Production process and production system of methionine and methionine hydroxyl analogue
A technology of methionine hydroxyl group and production process, which is applied in the field of feed preparation preparation, can solve the problems of difficult recovery of MET, long-term uniformity of high-concentration MHA, stability, fluidity, and impact on separation and recovery, so as to avoid impurities such as iminodinitrile , The reaction process is energy-saving and efficient, and the effect of increasing the reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
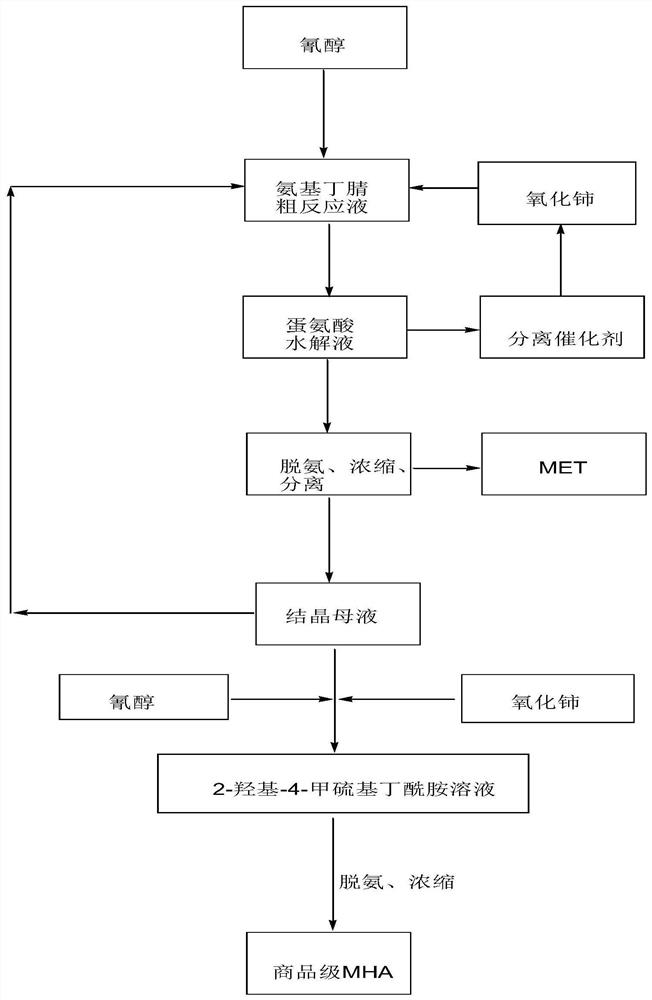
[0037] 364g cyanohydrin (72wt%, 2mol) and 1214g ammoniacal liquor (28wt%, 20mol) are sent into tubular reactor, at 65 ℃, react 10min under 0.8MPa condition, obtain 2-amino-4-methylthiobutyronitrile crude Reaction solution 1388g (the total concentration of aminobutyronitrile and cyanohydrin is about 18.87wt%, wherein 2-amino-4-methylthiobutyronitrile molar ratio is 88%, cyanohydrin molar ratio is 12%, iminoethanedinitrile is not detected out).
[0038] The above-mentioned 2-amino-4-methylthiobutyronitrile (aminobutyronitrile) crude reaction solution was transferred to a 3L reaction kettle, 681g of cerium oxide (2.6 times) was added, stirred and reacted at 100°C for 2h, and the total amount of cyanohydrin and aminobutyronitrile was analyzed. The residue is less than 0.1wt%. Solid-liquid separation and deamination are carried out while hot to obtain cerium dioxide and methionine hydrolyzate (MHA:MET=0.14, amide 0.15wt%). The methionine hydrolyzate is concentrated to obtain methio...
Embodiment 2
[0039] Embodiment 2 (reuse)
[0040] Mix 291.1g of cyanohydrin (90wt%, 2mol) and 637.5g of ammonia water (32%, 12mol) with a static mixer, then send them into a tubular reactor, and react at 75°C and 0.8MPa for 6min to obtain 2-amino- 4-methylthiobutyronitrile crude reaction solution 823.3g (the total concentration of aminobutyronitrile and cyanohydrin is about 31.83wt%, wherein 2-amino-4-methylthiobutyronitrile molar ratio is 87%, cyanohydrin molar ratio is 13% %, iminoethanedinitrile was not detected).
[0041] The above-mentioned 2-amino-4-methylthiobutyronitrile (aminobutyronitrile) crude reaction solution was transferred to a 3L reactor, and 472.3g of the crystallization mother liquor from Example 1 (MET: 3.19wt%, MHA: 7.63wt%, Amide: 0.26wt%) and 681g solid catalyst cerium oxide (2.6 times), 100 ° C stirring reaction for 2h, analysis of cyanohydrin and aminobutyronitrile total residue is less than 0.1wt%, while hot solid-liquid separation, deamination were obtained two ...
Embodiment 3
[0042] Embodiment 3 (reuse)
[0043] The same as in Example 2, after the ammonification of cyanohydrin, the crystallization mother liquor of Example 2 was reused, and reacted with cerium dioxide to obtain a methionine hydrolyzate (MHA:MET=0.41, amide 0.52wt%), and the methionine hydrolyzate was concentrated, separated, Drying and other steps Solid methionine 247.2g and crystallization mother liquor 577.3g (MET: 4.62wt%, MHA: 19.76wt%, amide: 0.71wt%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com