Nasal spray containing human urinary kallidinogenase and preparation method thereof
A technology for urinary kallikrein and nasal spray, which is applied in the directions of biochemical equipment and methods, medical preparations containing active ingredients, peptidase, etc. and other problems, to achieve the effect of easy absorption, improvement of cerebral infarction, and reduction of stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
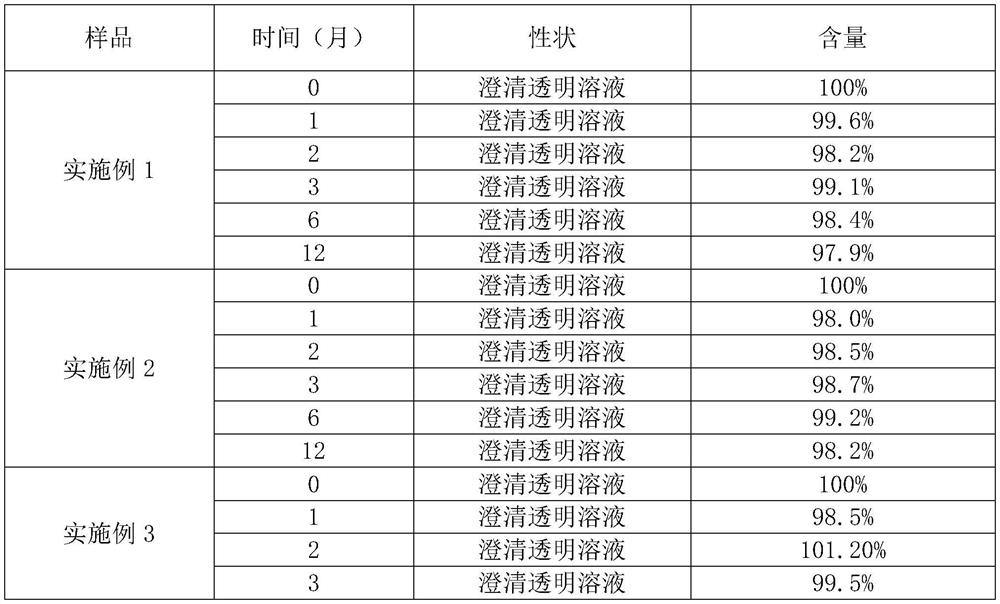
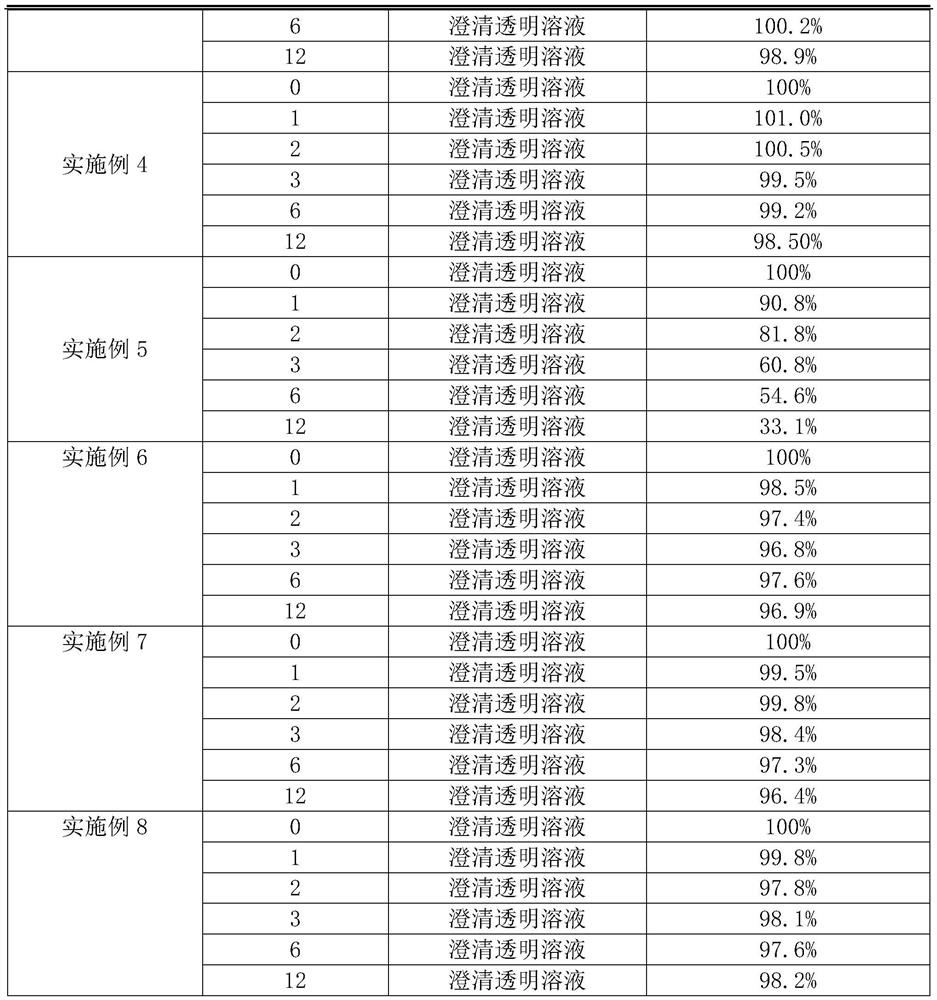
Embodiment 1
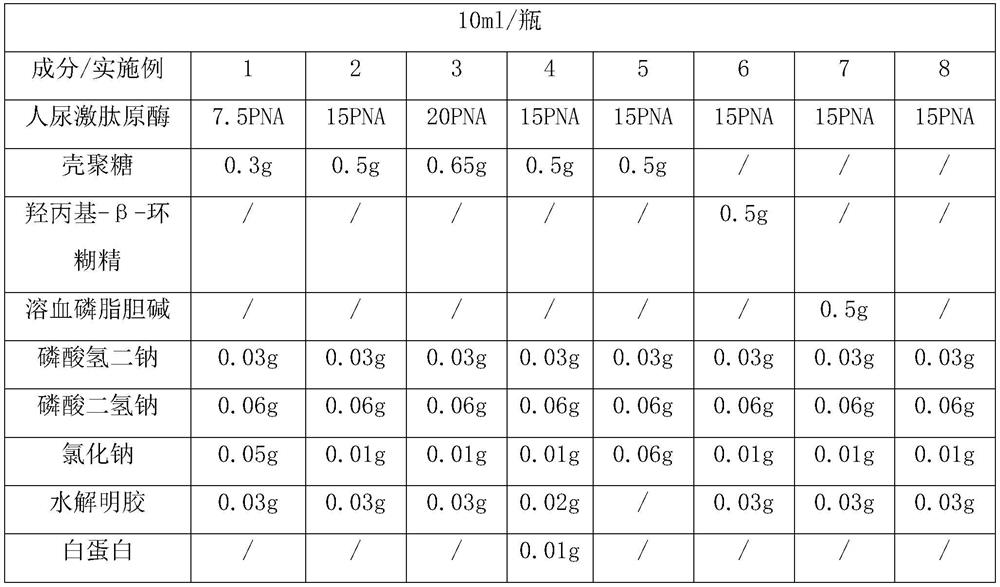
[0033] Human urinary kininogenase 750PNA, chitosan 30g, disodium hydrogen phosphate 3g, sodium dihydrogen phosphate 6g, sodium chloride 5g, hydrolyzed gelatin 3g, appropriate amount of hydrochloric acid and sodium hydroxide.
[0034] Preparation:
[0035] Step 1, the preparation of chitosan solution:
[0036] Take 800ml of water for injection, heat it to 30-40°C, add 3g of disodium hydrogen phosphate and 6g of sodium dihydrogen phosphate, stir to dissolve, then slowly add 30g of chitosan into it, stir until completely dissolved, and prepare a chitosan solution .
[0037] Step 2, preparation of nasal spray containing human urinary kininogenase:
[0038] Cool the chitosan solution in step 1 to 5-15°C, slowly add 750PNA human urinary kininogenase while stirring, stir until the solution is clear and transparent, then add 5g sodium chloride and 3g hydrolyzed gelatin, stir evenly, Add water for injection to 950ml, check the pH value of the liquid medicine, and adjust the pH value...
Embodiment 2
[0041] Human urinary kininogenase 1500PNA, chitosan 50g, disodium hydrogen phosphate 3g, sodium dihydrogen phosphate 6g, sodium chloride 1g, hydrolyzed gelatin 3g, appropriate amount of hydrochloric acid and sodium hydroxide.
[0042] Preparation:
[0043] Step 1, the preparation of chitosan solution:
[0044] Take 800ml of water for injection, heat it to 30-40°C, add 3g of disodium hydrogen phosphate and 6g of sodium dihydrogen phosphate, stir to dissolve, then slowly add 50g of chitosan into it, stir until completely dissolved, and prepare a chitosan solution .
[0045] Step 2, preparation of nasal spray containing human urinary kininogenase:
[0046]Cool down the chitosan solution in step 1 to 5-15°C, slowly add 1500PNA human urinary kininogenase while stirring, stir until the solution is clear and transparent, then add 1g sodium chloride and 3g hydrolyzed gelatin, stir evenly, Add water for injection to 950ml, check the pH value of the liquid medicine, and adjust the pH...
Embodiment 3
[0049] Human urinary kininogenase 2000PNA, chitosan 65g, disodium hydrogen phosphate 3g, sodium dihydrogen phosphate 6g, sodium chloride 1g, hydrolyzed gelatin 3g, appropriate amount of hydrochloric acid and sodium hydroxide.
[0050] Preparation:
[0051] Step 1, the preparation of chitosan solution:
[0052] Take 800ml of water for injection, heat it to 30-40°C, add 3g of disodium hydrogen phosphate and 6g of sodium dihydrogen phosphate, stir to dissolve, then slowly add 65g of chitosan into it, and stir until completely dissolved to obtain chitosan solution;
[0053] Step 2, preparation of nasal spray containing human urinary kininogenase:
[0054] Cool down the chitosan solution in step 1 to 5-15°C, slowly add 2000PNA human urinary kininogenase while stirring, stir until the solution is clear and transparent, then add 1g of sodium chloride and 3g of hydrolyzed gelatin, stir evenly, Add water for injection to 950ml, check the pH value of the liquid medicine, and adjust t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com