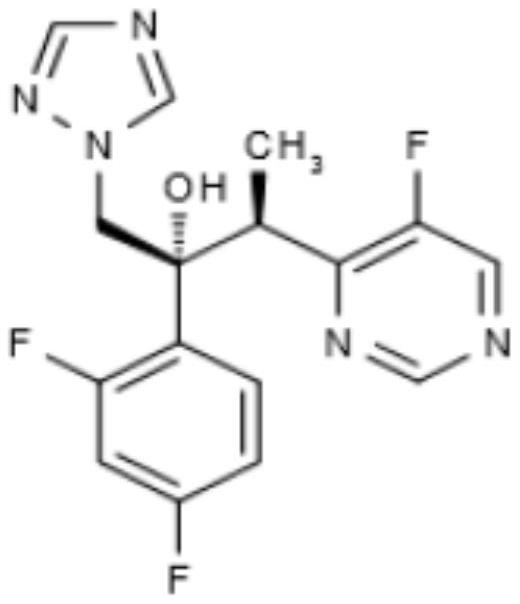
Voriconazole tablet and preparation method thereof
A technology for voriconazole tablets and tablet cores, which is used in pill delivery, antifungal agents, pharmaceutical formulations, etc., can solve problems such as inability to deduce the dosage of excipients and process parameters, slow dissolution rate, adverse reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] Preparation Process:
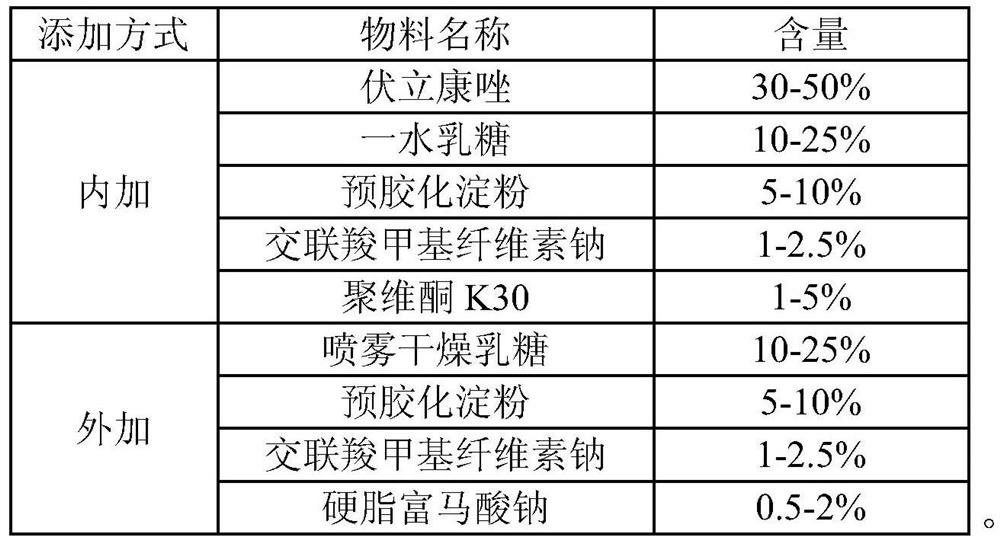
[0036] 1. Weigh the internally added material and add it to the high-speed stirring granulator, start stirring to make the mixture even;
[0037] 2. Prepare povidone K30 into a 5g / 100g aqueous solution. In 90-180s, add to the added material, high-speed stirring granulator to prepare soft material;
[0038] 3. The swing type granulator prepares 24 mesh granules;
[0039] 4. Use a fluidized bed to dry the obtained granules, control the air inlet temperature at 60-70°C, and keep the granule moisture at 3-5%;
[0040] 5. The dried granules are passed through a 20-mesh sieve for granulation;
[0041] 6. Granules are mixed evenly with external materials;
[0042] 7. Compressed into tablets;
[0043] 8. High-efficiency coating machine coating, coating weight gain 2.0-4.0%.
Embodiment 2
[0045]
[0046] Preparation Process:
[0047] 1. Weigh the internally added material and add it to the high-speed stirring granulator, start stirring to make the mixture even;
[0048] 2. Prepare povidone K30 into a 5g / 100g aqueous solution. In 90-180s, add to the added material, high-speed stirring granulator to prepare soft material;
[0049] 3. The swing type granulator prepares 24 mesh granules;
[0050] 4. Use a fluidized bed to dry the obtained granules, control the air inlet temperature at 60-70°C, and keep the granule moisture at 3-5%;
[0051] 5. The dried granules are passed through a 20-mesh sieve for granulation;
[0052] 6. Granules are mixed evenly with external materials;
[0053] 7. Compressed into tablets;
[0054] 8. High-efficiency coating machine coating, coating weight gain 2.0-4.0%.
Embodiment 3
[0056]
[0057]
[0058] Preparation Process:
[0059] 1. Weigh the internally added material and add it to the high-speed stirring granulator, start stirring to make the mixture even;
[0060] 2. Prepare povidone K30 into a 5g / 100g aqueous solution. In 90-180s, add to the added material, high-speed stirring granulator to prepare soft material;
[0061] 3. The swing type granulator prepares 24 mesh granules;
[0062] 4. Use a fluidized bed to dry the obtained granules, control the air inlet temperature at 60-70°C, and keep the granule moisture at 3-5%;
[0063] 5. The dried granules are passed through a 20-mesh sieve for granulation;
[0064] 6. Granules are mixed evenly with external materials;
[0065] 7. Compressed into tablets;
[0066] 8. High-efficiency coating machine coating, coating weight gain 2.0-4.0%.
[0067] Dissolution results
[0068] According to the dissolution test method (Chinese Pharmacopoeia 2020 edition general rule 0931 second method), the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com