Preparation of capecitabine intermediate
A capecitabine and intermediate technology, applied in the field of pharmaceutical synthesis, can solve problems such as low purity, achieve high yield and purity, save a lot of solvent extraction, and simplify post-processing operations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
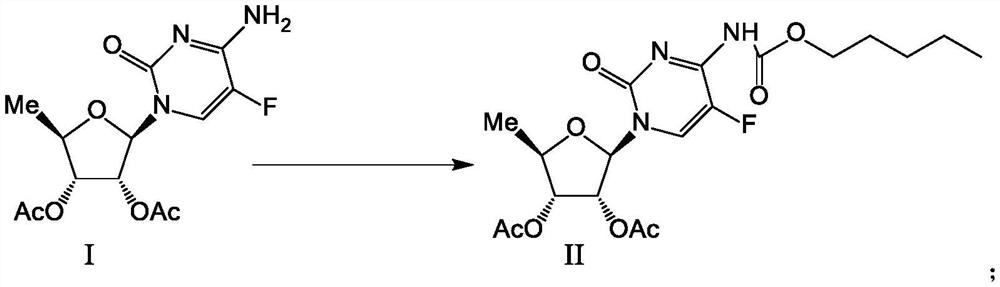
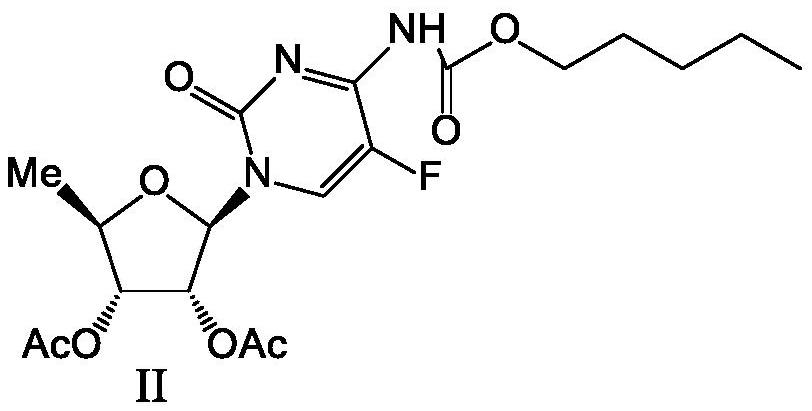
[0022] The preparation of formula II compound
[0023]
[0024] In the reaction flask, add 320 g of acetone, 200 g of the compound of formula I, and 132 g of pyridine, stir for 30 min, and cool down to -10~-5°C. 132 g of n-pentyl chloroformate was added dropwise, and the temperature was controlled at -10 to 0°C during the dropwise addition. After dropping, react at -5~5°C for 2 hours, and monitor by TLC until the reaction is completed. The reaction solution was added to the sodium chloride / purified water system (600g / 2200g) pre-cooled to below -5°C, then added dropwise with pre-cooled hydrochloric acid solution to adjust to neutrality, stirred below -5°C until solids were precipitated, and then -10 Stir at ~0°C for more than 4h. The filter cake was rinsed with 600 g of purified water at 0-5° C. to rinse and filter the filter cake to obtain 338 g of the wet product of the compound of formula II (equivalent to 267.0 g of dry product), and the purity was 99.7% as determined ...
Embodiment 2
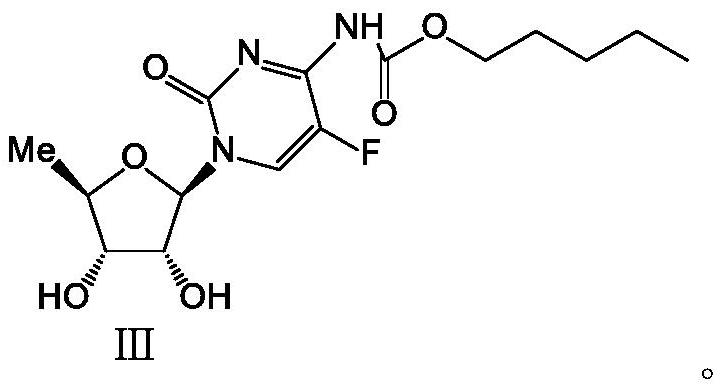
[0026] Preparation of formula Ⅲ compound capecitabine
[0027]
[0028] Throw about 338g of the wet product of the compound of the above formula II, 460g of purified water, stir, cool down to below -5°C, add dropwise an aqueous solution of sodium hydroxide (79g of sodium hydroxide is added to 673g of water), and control the temperature during the dropwise addition at -5 to 5°C. ℃. After dropping, keep the temperature at -5-5°C for 1 hour, and then track and monitor the mixture until the end of the reaction by TLC. Add hydrochloric acid water (hydrochloric acid / water: 76g / 76g) solution dropwise, adjust the pH to 6-7, after dropping, add 1200g of dichloromethane and stir for 10min, then add 200g of sodium chloride and stir for 10min, extract and separate. The organic phase was washed 3 times with purified water, each time using 480 g. The organic phase was concentrated to dryness under reduced pressure by controlling the temperature of the water bath at 35-40°C, adding 230 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com