Preparation method of nanogold colorimetric sensor and application of nanogold colorimetric sensor in detection of divalent nickel ions
A colorimetric sensor and nano-gold technology, applied in nanotechnology, nanotechnology, metal processing equipment, etc., can solve the problems of strict sample preservation conditions, high cost of analysis methods, and strong operation expertise, and achieve low cost and operability. The effect of strong sex and good application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Add 100mL of 0.24mM chloroauric acid tetrahydrate to a 250mL Erlenmeyer flask (soak overnight in fresh aqua regia, wash with ultrapure water, dry, and set aside), heat to boiling, add 4mL of 0.034M sodium phytate solution under magnetic stirring, Heat to reflux for 45-60 minutes, the solution turns from light yellow to wine red, stop heating, keep stirring to room temperature, and store at room temperature away from light.
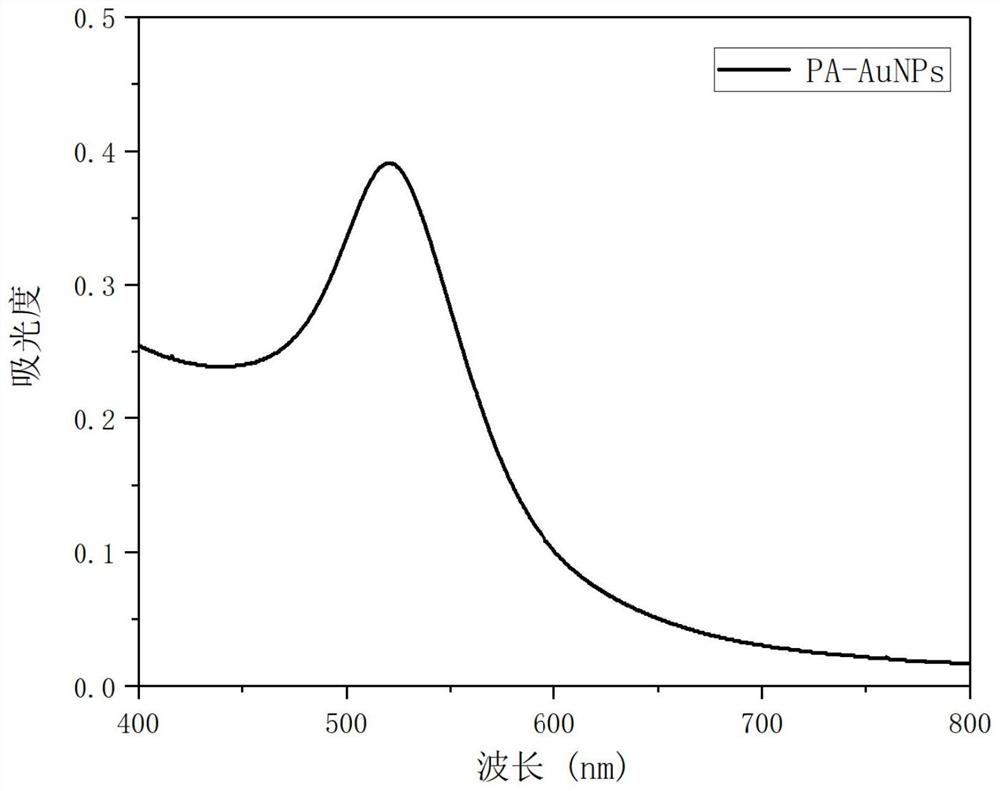
[0073] Measure the ultraviolet-visible absorption spectrum of the nano-gold solution modified by sodium phytate salt prepared in this embodiment, such as figure 1 shown. From figure 1 It can be seen that a characteristic absorption peak appears at 524 nm.
Embodiment 2
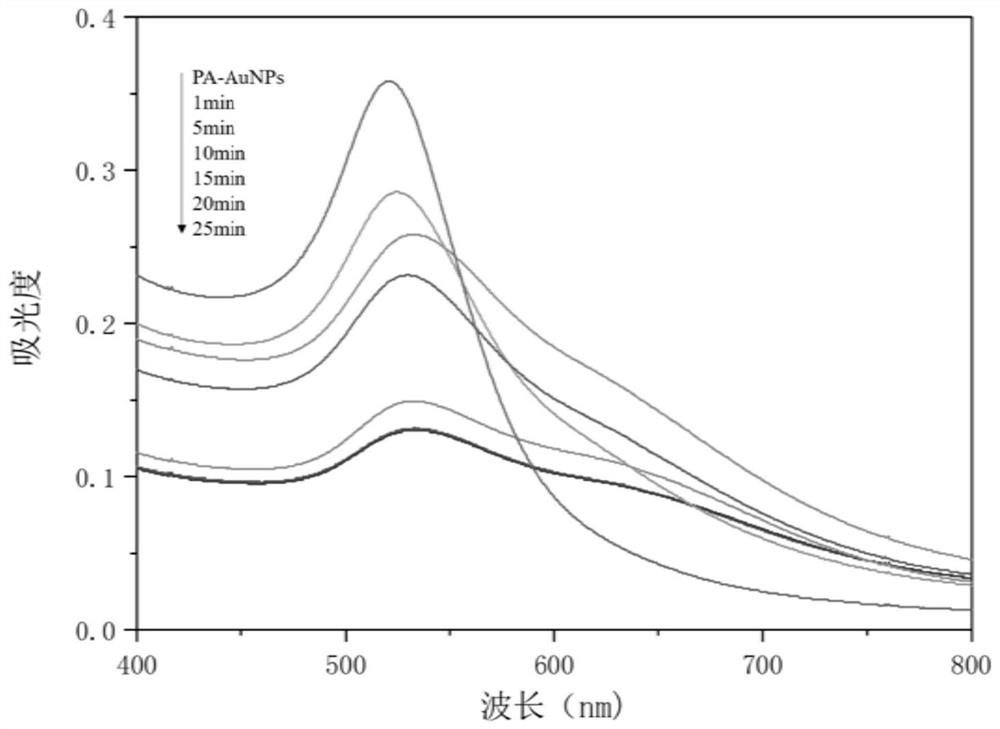
[0075] The phytic acid sodium salt modified nano-gold colorimetric sensor prepared above was mixed with an equal volume of 1×10 -5 The nickel chloride aqueous solution of M is mixed, and uses ultraviolet-visible spectrometer (Uv-Vis) to detect the change of corresponding ultraviolet-visible spectrum in 1~25min, and experimental result is as follows figure 2 shown. From figure 2 It can be seen that the UV-visible absorption peak of the nano-gold solution began to decrease significantly at 1 min.
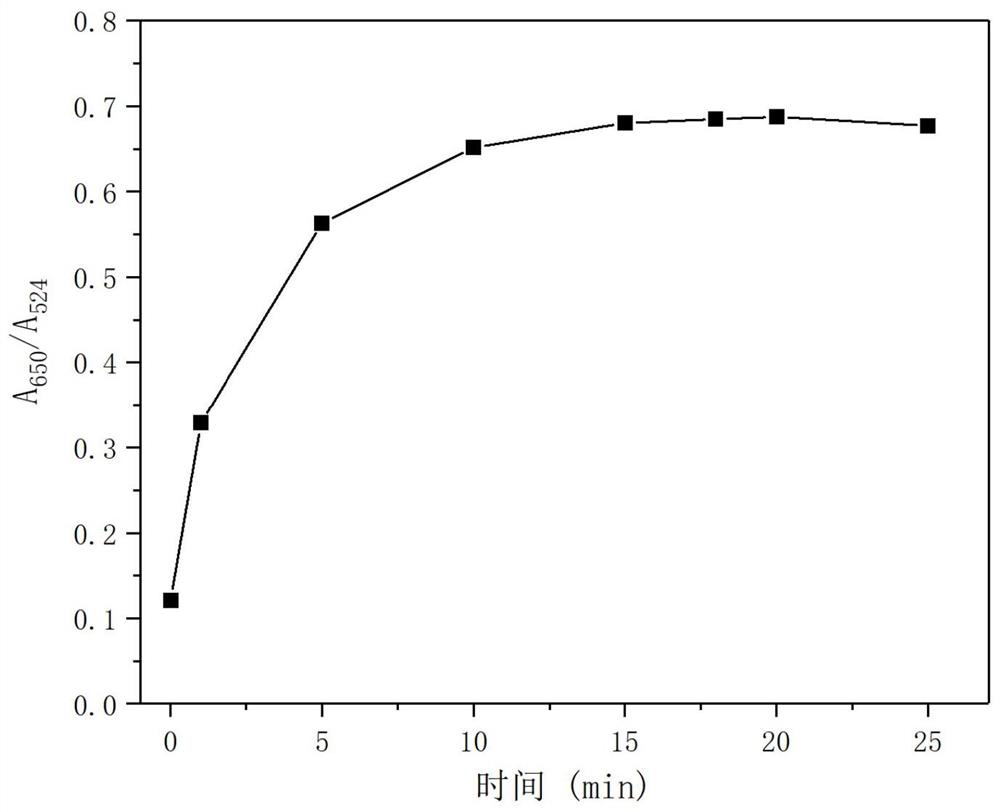
[0076] Observe the ratio of the absorption value corresponding to the characteristic absorption wavelength (A 650 / A 524 ) changes with time, such as image 3 shown. From image 3 It can be seen that the value is basically stable after 15 minutes, so the nano-gold probe modified by sodium phytate can quickly detect Ni 2+ .
Embodiment 3
[0078] The nano-gold colorimetric sensor modified by sodium phytate salt prepared in Example 1 was used for 1×10 -5 M Ni 2+ For detection, take 1.5mL concentration as 2×10 -5 M nickel chloride aqueous solution, and then measure 1.5mL containing other metal ions (FeCl 3 , FeCl 2 ,KCl,CuCl 2 ,MnCl 2 ,CdCl 2 ,CoCl 2 ,NiCl 2 ,Pb(NO 3 ) 2 ,ZnCl 2 ,MgCl 2 ,CaCl 2 ,AlCl 3 ,Cr 2 O7 2- ) concentration is 4×10 -5 M aqueous solution, the above-mentioned nano-gold modified by sodium phytate salt was mixed with the solution containing the above-mentioned metal ions, and 100 μL of 0.2M acetic acid-sodium acetate buffer solution (pH=3.6) was added, and the color change was observed. The experimental results were as follows: Figure 4 shown. It was found that only Ni 2+ The nano-gold solution turns from red to blue.
[0079] Simultaneously, measure the ultraviolet-visible absorption spectrum of the nanogold modified by phytic acid sodium salt and the mixed solution containi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com