A kind of construction method of fcrn gene humanized animal model
A humanized and genetic technology, applied in the field of animal genetic engineering and genetic modification, can solve the problems of clinical differences in antibody half-life, achieve the effect of increasing the content of drug evaluation and solving clinical differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: FcRn humanization targeting homologous DNA donor construction
[0042] Homologous recombination was used to replace the coding region of the extracellular region of the human FCRN gene with the coding region of the extracellular region of the C57BL / 6 mouse FcRn gene, and the UTR sequence of the C57BL / 6 mouse was retained to construct a targeting vector containing the C57BL / 6 background. Sequence shown in SEQ ID NO: 1: wherein 1-782bp is the C57BL / 6 mouse-derived 5'UTR coding region and homology arm sequence, including exon1, intron1, and part of exon2 in turn; 783-2838bp is the human FCRN gene part, including part in turn Sequences of Exon2, Intron2, Exon3, Intron3, Exon4, Exon5, Intron5, and part of Exon6; the 2839-3727bp part is the C57BL / 6 mouse FcRn region, 3'UTR and homology arm sequences, including parts of exon 6, intron6, and exon7 in turn .
[0043] SEQ ID NO: 1
[0044]ggctggacagaggcccaaggggtgtgcttaggagctagtgggtggagttggatgccctcagagttctccagtcctaact...
Embodiment 2
[0045] Example 2 FcRn humanized mouse sgRNA design
[0046] Determine the approximate region of sgRNA based on the replacement fragments. Use the sgRNA evaluation software to select the sequences with the lowest off-target rate for the target fragment, and the following sequences are used as the candidate sgRNAs. Design and synthesis recognize the 5' end target site and 3' end target site, and construct the sgRNA expression vector. The sgRNA recognition sites at both ends are located on the first and fifth exons of the mouse Tnfrsf18 gene, respectively.
[0047] sgRNA transcription preparation method: using PrimerStar or PrimerStar Max system, sgRNA-F and sgRNA-R as primers, and the puc57-sgRNA plasmid (1:30 dilution) with the correct sequencing as the template to carry out PCR, the PCR product is purified, and the sgRNA transcription preparation template is prepared . Transcription of sgRNA was performed using the T7-ShortScript in vitro transcription kit (AM1354).
[004...
Embodiment 3F
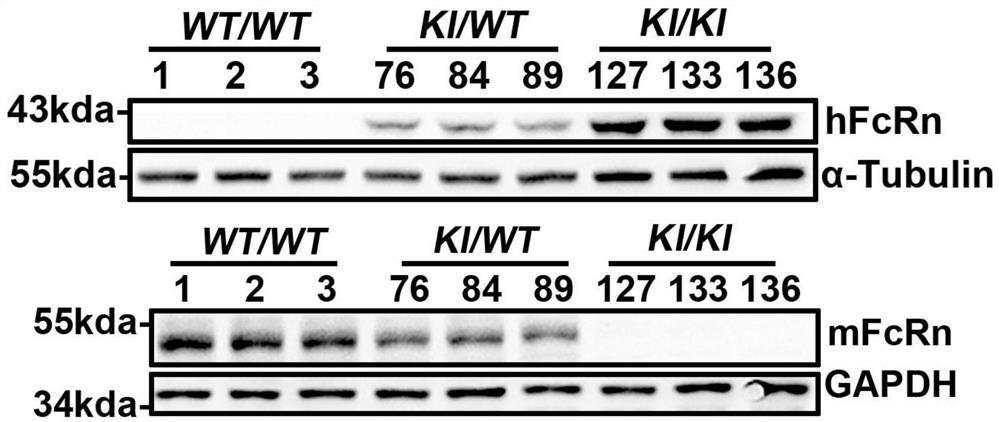
[0052] Example 3 Establishment of FcRn humanized mouse model
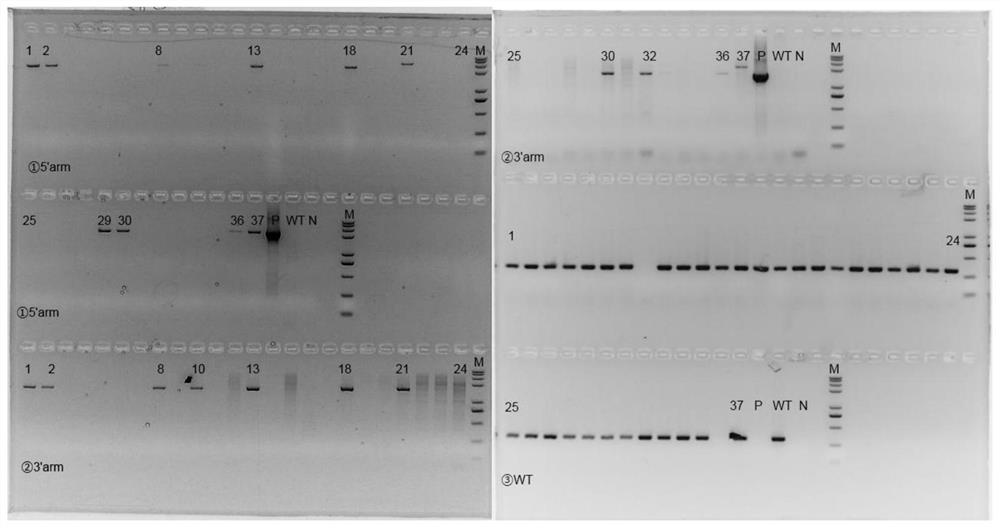
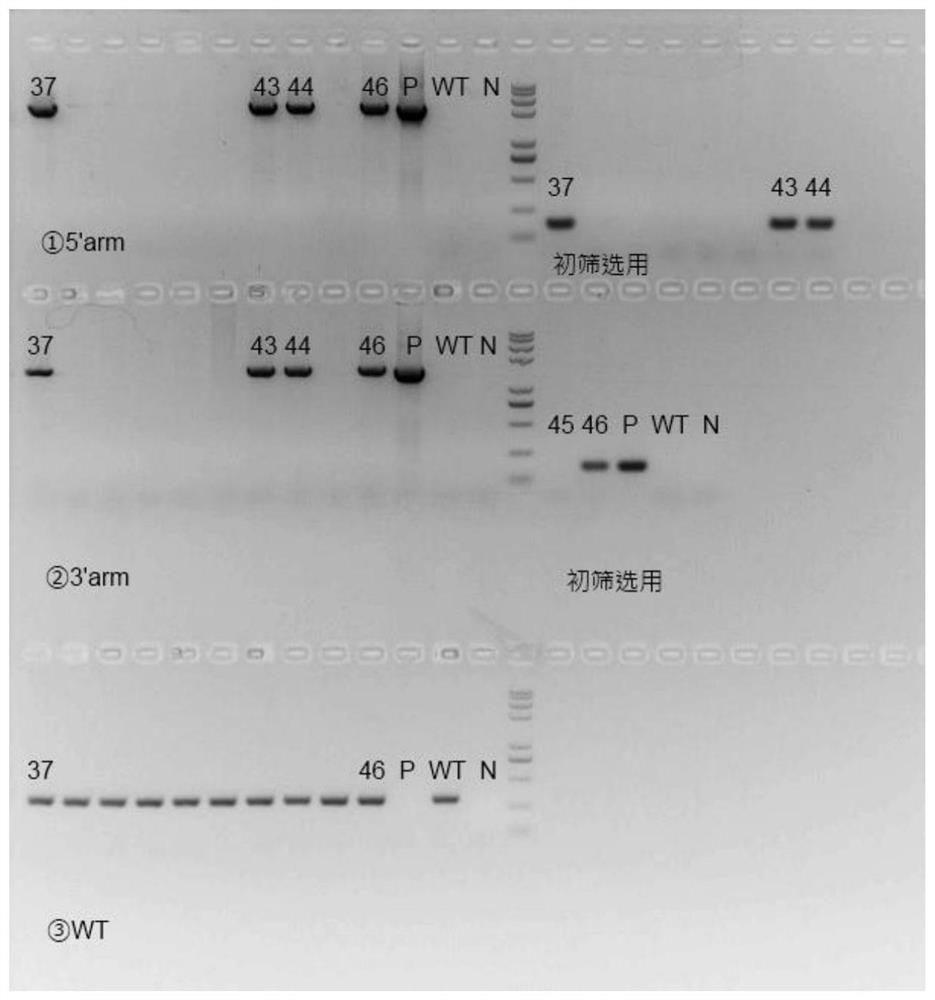
[0053] The sgRNA designed in Example 2 and the FcRn humanized targeting homologous DNA donor designed in Example 1 were mixed with Cas9 protein, injected into 0.5-day-old mouse fertilized eggs, and transplanted into 0.5-day-old pseudopregnant female mice. , After the mice were born, the target mice (F0) were screened out by genetic identification.
[0054] Genotype identification of the F0 generation of humanized mice: The obtained mouse tail genomic DNA of F0 mice was identified by PCR on both ends of the target after hitting the target, primers 710181-FCRN-5tF1 / 710181-hFCRN-5tR1 respectively It is located outside the 5' homology arm and within the human fragment of the targeting vector. If the pair of primers is amplified to produce a PCR product, it means that the target vector has been effectively inserted at the 5' of the mouse genome; 710181-hFCRN-3tF1 / 710181-FCRN- 3tR1 is located in the human segment of the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com