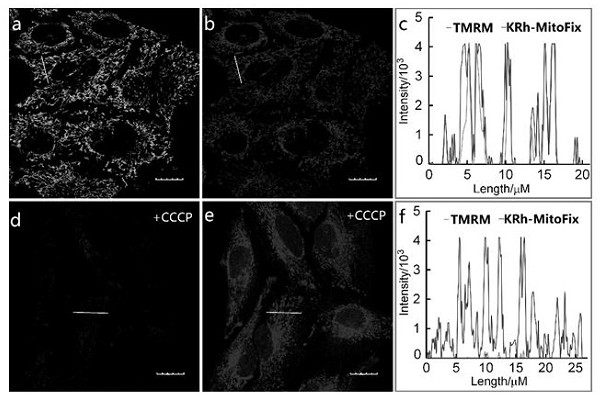
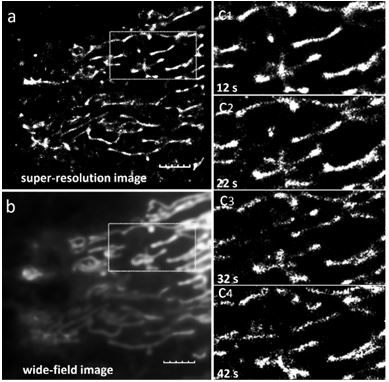
Near-infrared fluorescent compound with targeting function and application thereof
A technology of fluorescent compounds and compounds, applied in the fields of organic chemistry, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of fine research on the microstructure of difficult organisms, lack of functional dye compounds, and difficult super-resolution imaging.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] i) Under argon, A (2.97 mmol), Pd(OAc) 2 (0.45 mmol), BINAP (0.32 mmol), Cs 2 CO 3 (8.62 mmol) and pyrrolidine (1.2 mL) were dissolved in toluene and reacted with stirring at 110°C. The reaction was monitored by TLC until the reaction was complete. After cooling to room temperature, the reaction mixture was washed with water and evaporated. The crude product was purified by silica gel column chromatography (ethyl acetate / hexane = 1 / 25) to obtain pale yellow solid B1.
[0033] ii) Use a water separator to remove water, stir the mixture of compound B1 (0.94 mmol), ethylene glycol (2 mL), and TsOH (0.29 mmol), react at 150 ° C, monitor the reaction with TLC until the reaction of compound B1 is complete. The reaction mixture was concentrated and purified by silica gel column chromatography to obtain pale yellow solid C1.
[0034] iii) Compound C1 (0.91 mmol) was added to 5 mL of DMF and stirred at room temperature. NBS (1.82 mmol) was added to the reaction...
Embodiment 2
[0038]
[0039] i) Under argon, A (2.97 mmol), Pd(OAc) 2 (0.45 mmol), BINAP (0.32 mmol), Cs 2 CO 3 (8.62 mmol) and diethylamine (1.3 mL) were dissolved in toluene and reacted with stirring at 120°C. The reaction was monitored by TLC until the reaction was complete. After cooling to room temperature, the reaction mixture was washed with water and evaporated. The crude product was purified by silica gel column chromatography (ethyl acetate / hexane = 1 / 25) to obtain light yellow solid B2.
[0040] ii) Use a water separator to remove water, stir the mixture of compound B2 (0.94 mmol), ethylene glycol (2 mL), and TsOH (0.29 mmol), react at 150 ° C, monitor the reaction with TLC until the reaction of compound B2 is complete. The reaction mixture was concentrated and purified by silica gel column chromatography to obtain light yellow solid C2.
[0041] iii) Compound C2 (0.91 mmol) was added to 5 mL of DMF and stirred at room temperature. NBS (1.82 mmol) was added to the react...
Embodiment 3
[0045]
[0046] i) Under argon, A (2.97 mmol), Pd(OAc) 2 (0.45 mmol), BINAP (0.32 mmol), Cs 2 CO 3 (8.62 mmol) and cyclohexylamine (1.4 mL) were dissolved in toluene, and the reaction was stirred at 115°C, and the reaction was monitored by TLC until the end of the reaction. After cooling to room temperature, the reaction mixture was washed with water and evaporated. The crude product was purified by silica gel column chromatography (ethyl acetate / hexane = 1 / 25) to obtain pale yellow solid B3.
[0047] ii) Use a water trap to remove water, stir a mixture of compound B3 (0.94 mmol), ethylene glycol (2.1 mL), and TsOH (0.29 mmol), react at 150°C, and monitor the reaction with TLC until compound B3 is completely reacted. The reaction mixture was concentrated and purified by silica gel column chromatography to obtain light yellow solid C3.
[0048] iii) Compound C3 (0.91 mmol) was added to 5 mL of DMF and stirred at room temperature. NBS (1.82 mmol) was added to the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com