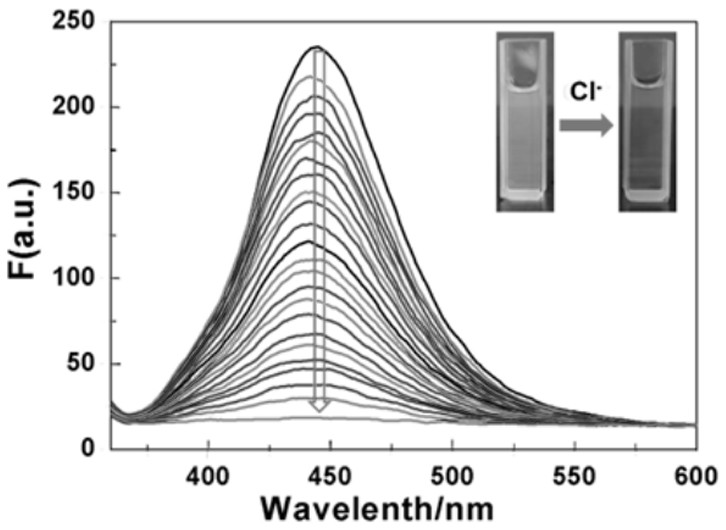
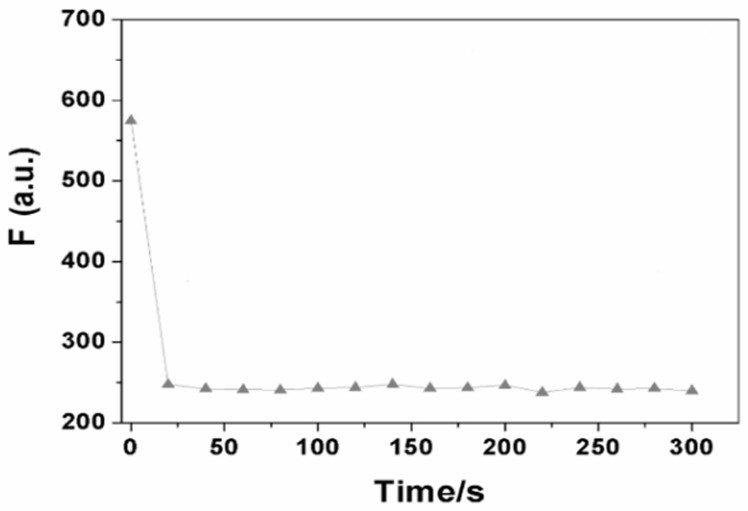
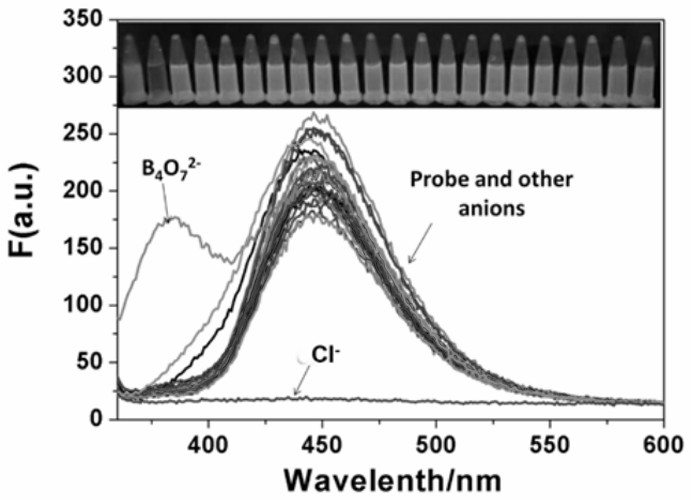
A fluorescent probe for recognizing chloride ions and its preparation method and application
A fluorescent probe, chloride ion technology, used in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of limiting anion species and fluorescence response sensitivity, achieve strong resistance to other molecular interference, post-processing process Simple, selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of the above-mentioned fluorescent probe for recognizing chloride ions comprises the following steps:
[0029] Step 1, 6-methoxyquinoline was dissolved in C 2 h 5 OH to obtain the first solution, 3-bromopropylamine hydrobromide dissolved in CH 3 The second solution was obtained in CN; the above two solutions were mixed and heated to a reaction temperature of 75-85°C, and the crude product obtained after the reaction was purified and separated to obtain the compound represented by formula (3);
[0030] .
[0031] The reaction formula is expressed as:
[0032]
[0033] Step 2, dissolving the compound represented by formula (3) obtained in step 1 and benzenesulfonyl chloride in dichloroethane, and separating and purifying after completion of the stirring reaction to obtain the target probe compound represented by formula (4).
[0034] The reaction formula is expressed as:
[0035]
[0036] Relatively specifically, the above-mentioned met...
Embodiment 1
[0045] Step 1, the synthesis of the compound shown in formula (3):
[0046] Dissolve 6-methoxyquinoline (10.0 mmol, 1.59 g) in 10 mL C 2 h 5 In OH, 3-bromopropylamine hydrobromide (10 mmol, 2.19 g) was dissolved in 2 mL CH 3 In CN, the above two solutions were mixed, heated to a reaction temperature of 80°C, and the reaction time was 6 hours, a white precipitate was formed, the crude product was centrifuged at 8000 rpm for 5 min, the precipitate was collected, and washed with C 2 h 5 Wash with OH until there is no 6-methoxyquinoline in the supernatant, and vacuum-dry the white precipitate to obtain the pure compound of formula (3), with a yield of 84%.
[0047] Step 2, the synthesis of the target probe compound:
[0048] Dissolve 1.00 mmol of the compound of formula (3) (0.203 g) and 1.00 mmol of benzenesulfonyl chloride (0.176 g) in 10 mL of dichloroethane, stir and react for 4 h, filter the precipitate in the system and recrystallize with ethanol to obtain pure Product,...
Embodiment 2
[0050] The difference from Example 1 is only that in step 1, the reaction temperature is 75° C., and the reaction time is 8 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com