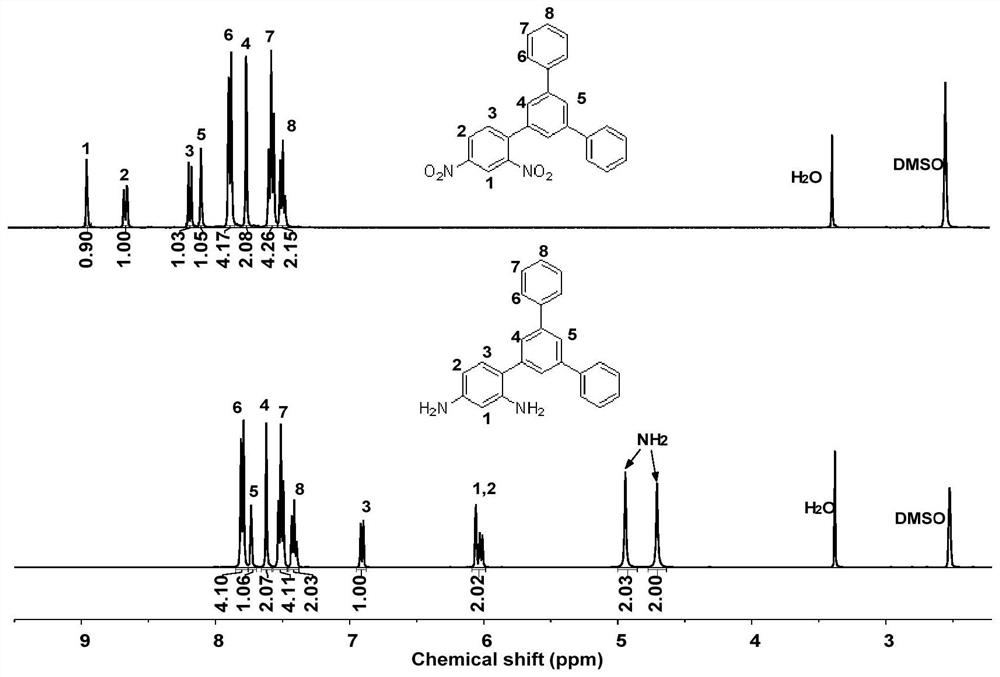
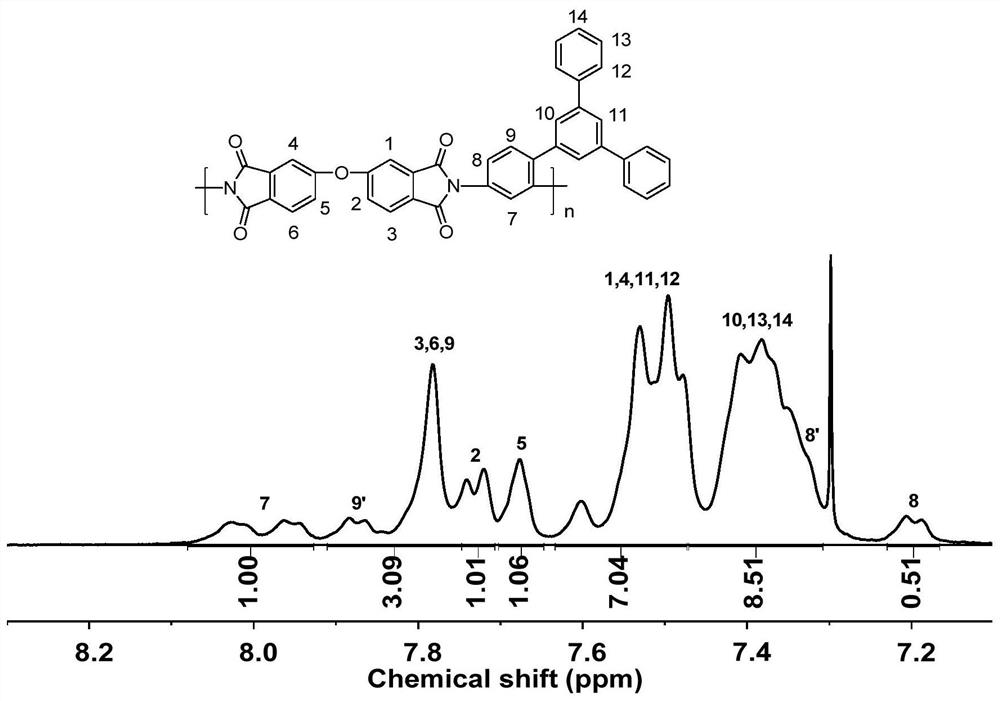
Asymmetric aromatic diamine monomer containing terphenyl large substituent side groups and polyimidecontaining terphenyl large substituent side groups
A polyimide, aromatic diamine technology, applied in the preparation of amino compounds, the preparation of organic compounds, organic chemistry and other directions, can solve the problems of low molar polarizability, thermal stability and mechanical property loss, and achieve easy The effect of industrialization, easy purification and separation, and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Preparation of asymmetric aromatic diamine monomers containing terphenyl large substituted side groups
[0053] (a) Under nitrogen protection, 19.76g (8mmol) of 2,4-dinitrobromobenzene, 24.12g (88mmol) of 3,5-diphenylboronic acid, 1.85g (0.16mmol) of four ( Triphenylphosphine) palladium joins in the there-necked flask of 500mL, adds respectively 150mL volume ratio and is the mixed solvent of 2:1 toluene and ethylene glycol dimethyl ether and the sodium carbonate solution of 120mL 1mol / L, heats up after stirring at room temperature for half an hour Reaction at 100°C for 8h, after the reaction was completed, the intermediate compound 3,5-(diphenyl)phenyl-2,4-dinitrobenzene was obtained by suction filtration, drying and further recrystallization, with a yield of 91% ( Here the yield is obtained by the ratio of the mass of the intermediate compound actually obtained to the mass of the intermediate compound obtained theoretically).
[0054] (b) Add 18.82g (0.05mol) of t...
Embodiment 2
[0058] (1) Preparation of aromatic diamine monomer containing terphenyl large substituted side group asymmetric structure
[0059] (a) Under nitrogen protection, 19.76g (8mmol) of 2,4-dinitrobromobenzene, 24.12g (88mmol) of 3,5-diphenylphenylboronic acid, 2.10g (0.18mmol) of four ( Triphenylphosphine) palladium joins in the there-necked flask of 1000mL, adds respectively 320mL volume ratio is the mixed solvent of 4:1 toluene and ethylene glycol dimethyl ether and the sodium carbonate solution of 240mL 2mol / L, heats up after stirring at room temperature for half an hour Reaction at 110°C for 7h, after the reaction was completed, the intermediate compound 3,5-(diphenyl)phenyl-2,4-dinitrobenzene was obtained by suction filtration, drying and further recrystallization, with a yield of 88% ( Here the yield is obtained by the ratio of the mass of the intermediate compound actually obtained to the mass of the intermediate compound obtained theoretically).
[0060] (b) Add 18.82g (0....
Embodiment 3
[0064] (1) Preparation of aromatic diamine monomer containing terphenyl large substituted side group asymmetric structure
[0065] (a) Under nitrogen protection, 14.82g (6mmol) of 2,4-dinitrobromobenzene, 18.09g (66mmol) of 3,5-diphenylboronic acid, 1.39g (0.13mmol) of four ( Triphenylphosphine) palladium joins in the there-necked flask of 500mL, adds respectively 250mL volume ratio and is the mixed solvent of 3:1 toluene and ethylene glycol dimethyl ether and the sodium carbonate solution of 150mL 2mol / L, heats up after stirring at room temperature for half an hour Reaction at 100°C for 10h, after the reaction was completed, the intermediate compound 3,5-(diphenyl)phenyl-2,4-dinitrobenzene was obtained by suction filtration, drying and further recrystallization, with a yield of 89% ( Here the yield is obtained by the ratio of the mass of the intermediate compound actually obtained to the mass of the intermediate compound obtained theoretically).
[0066] (b) Add 10.16g (0.03...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com