Preparation method of ibrutinib
A technology of ibrutinib and compounds, applied in the field of preparation of ibrutinib, can solve the problems of long reaction route, not environmental protection, and many wastes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
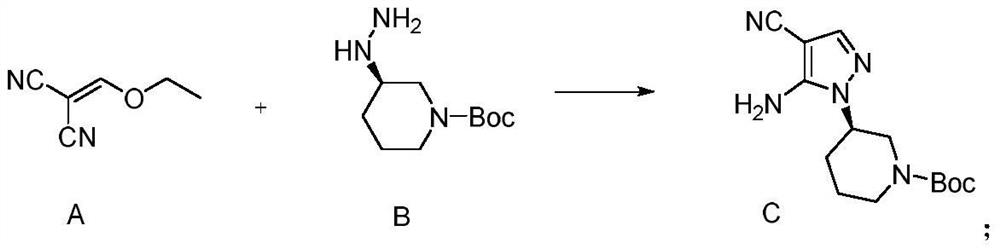
[0082] The preparation of embodiment 1 compound C
[0083]
[0084] Add 0.61kg of compound A, 0.1L of absolute ethanol, and 1.08kg of compound B into the reaction kettle at room temperature, and heat up to 90°C for reflux reaction after the addition. After 5 hours, the HPLC results show that the raw materials have reacted completely, stop the reaction, and cool to room temperature , the reaction solution was filtered, and the filter cake was dried at 80° C. for 14.0 h to obtain 1.32 kg of solid, with a yield of 87.1% and a purity of 99.5%.
[0085] After testing: MS: [M+1] = 304.2, NMR 1 H NMR(400MHz,DMSO-d6)δ8.23(s,1H),6.31(m,2H),3.85-3.91(m,2H),3.96(t,1H),3.52-3.56(m,2H), 1.87-2.12(m,2H),1.58-1.68(m,2H),1.39(s,9H).
Embodiment 2
[0086] The preparation of embodiment 2 compound C
[0087] Add 0.30kg of compound A, 50mL of absolute ethanol, 0.54kg of compound B to the reaction kettle at room temperature, add 0.505kg of triethylamine, and heat up to 90°C for reflux reaction after the addition. After 4 hours, the HPLC results show that the raw materials are completely reacted. Stop the reaction, cool to room temperature, filter the reaction solution, and dry the filter cake at 80° C. for 14.0 h to obtain Compound C, 0.69 kg in total, with a yield of 91.2% and a purity of 99.3%.
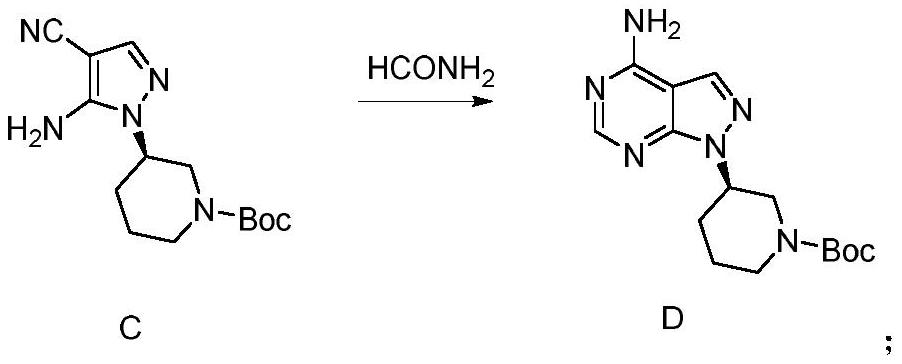
Embodiment 3
[0088] The preparation of embodiment 3 compound D
[0089]
[0090] Add 0.43 kg of compound C and 5 mL of formamide to the reaction bottle at room temperature, heat up to 80°C to 85°C for reflux reaction after addition, and react for 12 hours. After the HPLC results show that the raw materials are completely reacted, stop the reaction, cool to room temperature, and add water After stirring, filtering, the filter cake was dissolved in chloroform, concentrated, and purified by methanol-chloroform gradient silica gel chromatography to obtain compound D, 0.29 kg in total, with a yield of 64.5% and a purity of 98.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com