Mutated piggybac transposase
A technology of transposase and amino acid, applied in the direction of transferase, enzyme, hydrolase, etc., can solve the problems of high R&D cost, delay and inefficiency, and long development lead time of biological drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0187] Example 1 Identification and Testing of Mutations
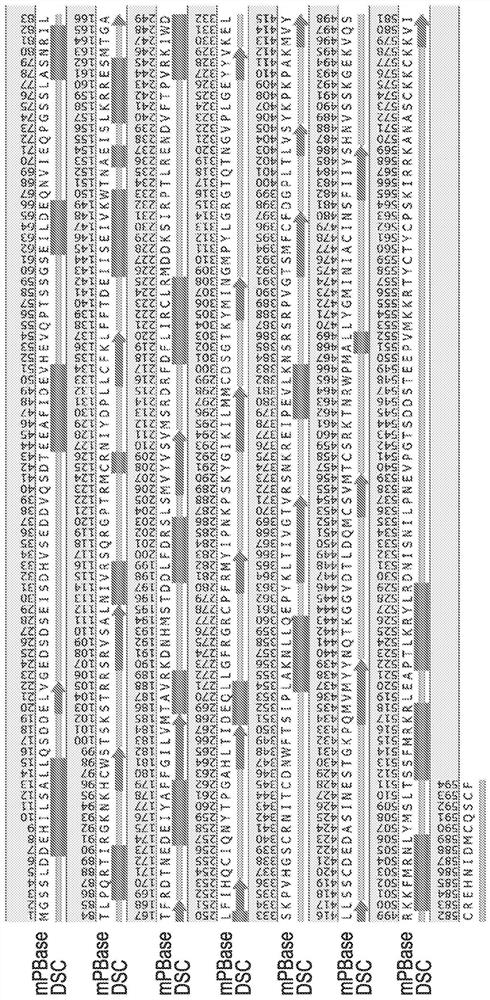
[0188] The structure of piggyBac transposase and other related transposases is unknown. We predicted wild-type Trichoplusia piggyBac transposase (SEQ ID NO: 2) The secondary structure motif. see figure 1 . We found that the piggyBac transposase is predominantly an α-helical protein. We designed mutations that might stabilize the α-helix, and these mutations could improve the overall stability of the transposase, which in turn could positively affect the expression of the transposase. We identified I147, I176, I221, I247 as residues that could potentially be mutated to improve alpha-helix stability of piggyBac transposase.
[0189] We also identified putative N-linked glycosylation sites (ie, NXS / T motifs) in the transposase sequence. Since, in general, NXT motifs undergo more complete glycosylation compared to NXS motifs, we hypothesized that mutation of NXS motifs to NXT motifs might improve overall glycosylatio...
example 2
[0198] Example 2 Expression of double and triple transposase mutants in MSX-added GS KO host cells
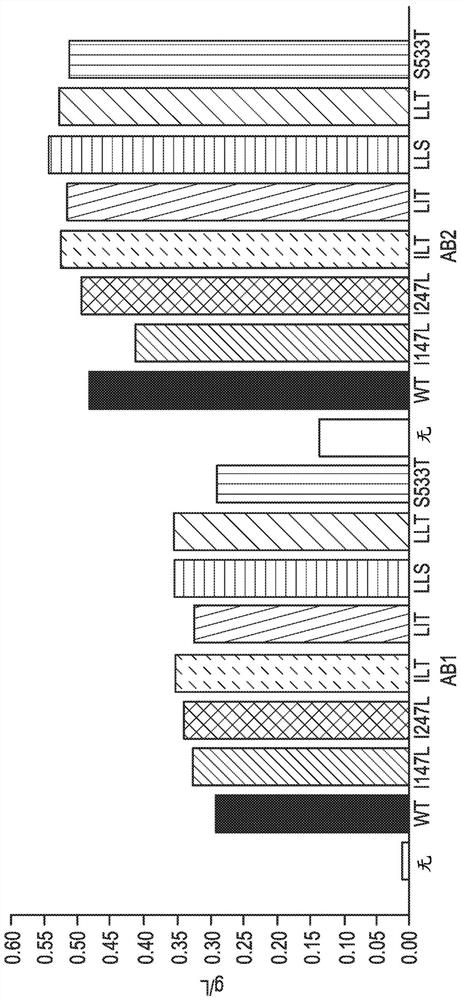
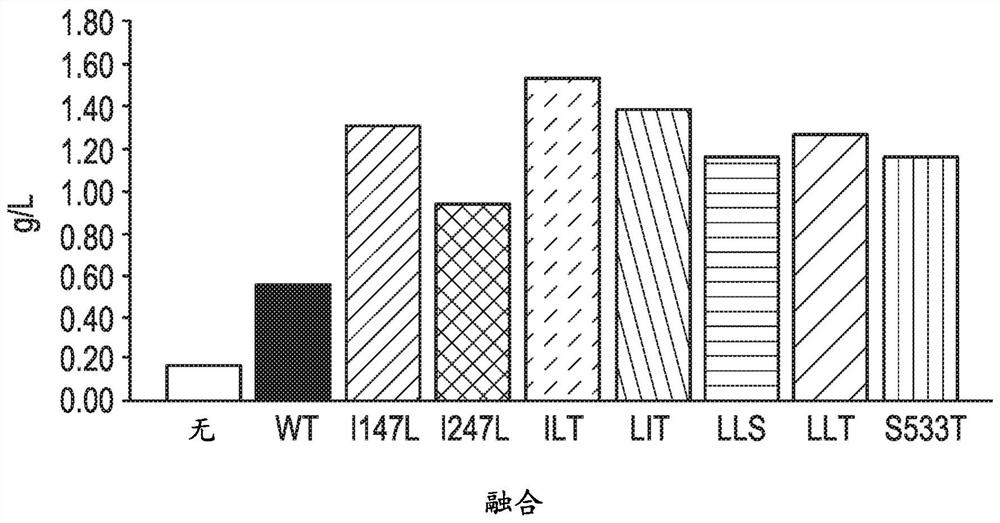
[0199] Using electroporation (Bio-Rad, Hercules, CA) DNA piggyBac transposase encoding 1) double mutant "ILT" (ITR, SEQ ID NO: 11), 2) triple mutant Glutamine synthase knockout CHO cells (GSKO cells) were transfected with a "LLT" DNA piggyBac transposase (LLT, SEQ ID NO:9) and 3) a circular plasmid not encoding piggyBac transposase (None), all Both cases were combined with a circular plasmid containing the gene of interest and the 5' and 3' inverted repeat elements of the Trichoplusia piggyBac transposon. Three target genes were tested, bispecific T cell engager heterologous Fc (Tcell engager heteroFc), IgG-scFv and monoclonal antibody (mAb).
[0200] On day 3 after transfection (25), after initial pools had recovered >90% viability (0-25) or not at all (0), 25 μM of methionine sulfoximine (MSX) (EDM dense EDM Millipore, Burlington, Mass.). The addition of methionine sulfoxi...
example 3
[0205] Example 3 Transfection using DNA or mRNA piggyBac transposase.
[0206] The transposase used to integrate the gene of interest can be DNA or mRNA based. One concern with using transposases for DNA transcription is the potential for integration of the transposase gene into the genome, which could result in active transcription / translation of the transposase in the transfected cell line. This could lead to genome instability due to transposase activity at potential recessive transposase recognition sites. An alternative is to use mRNA for transfection since it does not integrate into the genome.
[0207] Unmodified mRNA transcripts using wild-type bases and with 25% pseudo-U and 5-methyl-C end-capping substitutions were prepared against the piggyBac transposase of double mutant "ILT" and triple mutant "LLT" modification of synthetic mRNA transcripts.
[0208] Two sets of experiments were performed to evaluate mRNA translation transposases in transfections. In a first ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com