Novel chemiluminescent substrates for factor xa
A chemiluminescence, factor technology, applied in the fields of organic chemistry, bulk chemical production, biochemical equipment and methods, etc., can solve problems such as discomfort measurement, continuous measurement of discomfort, reduction of physiological conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] Example 1 - Providing a substrate of the invention
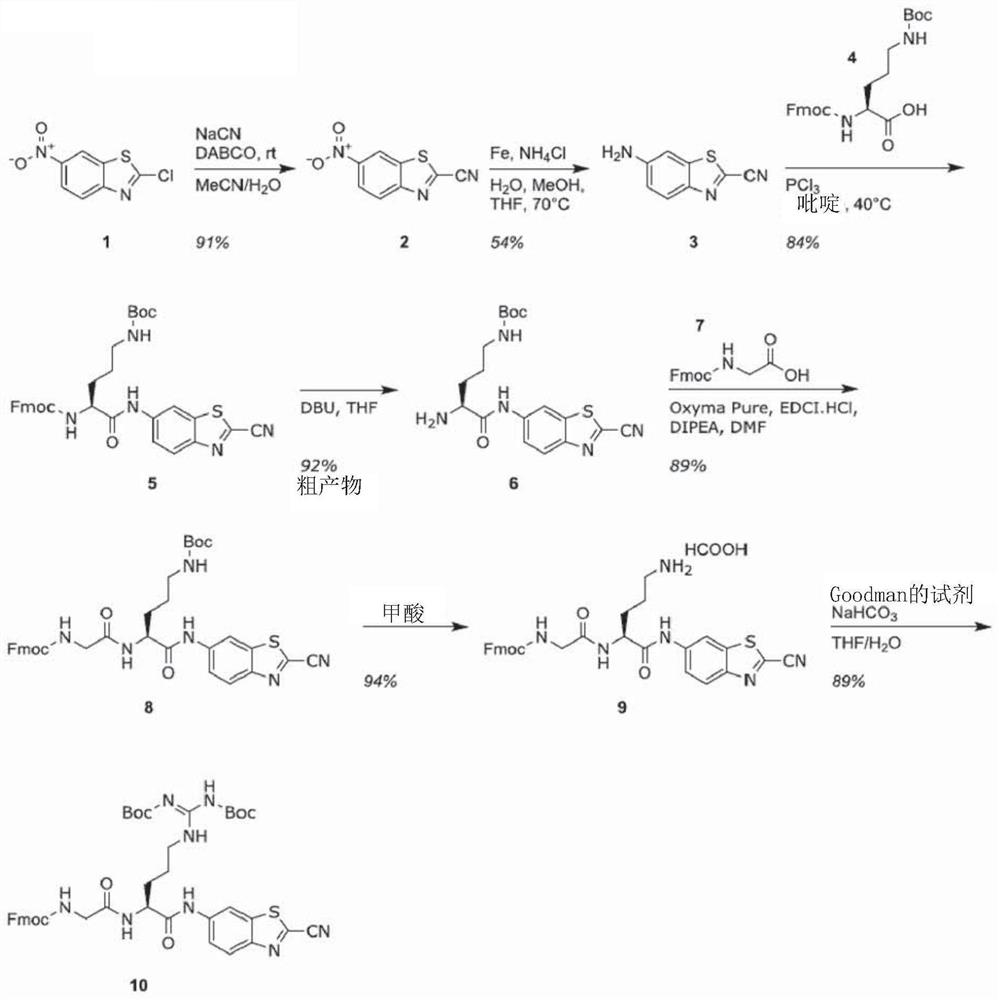
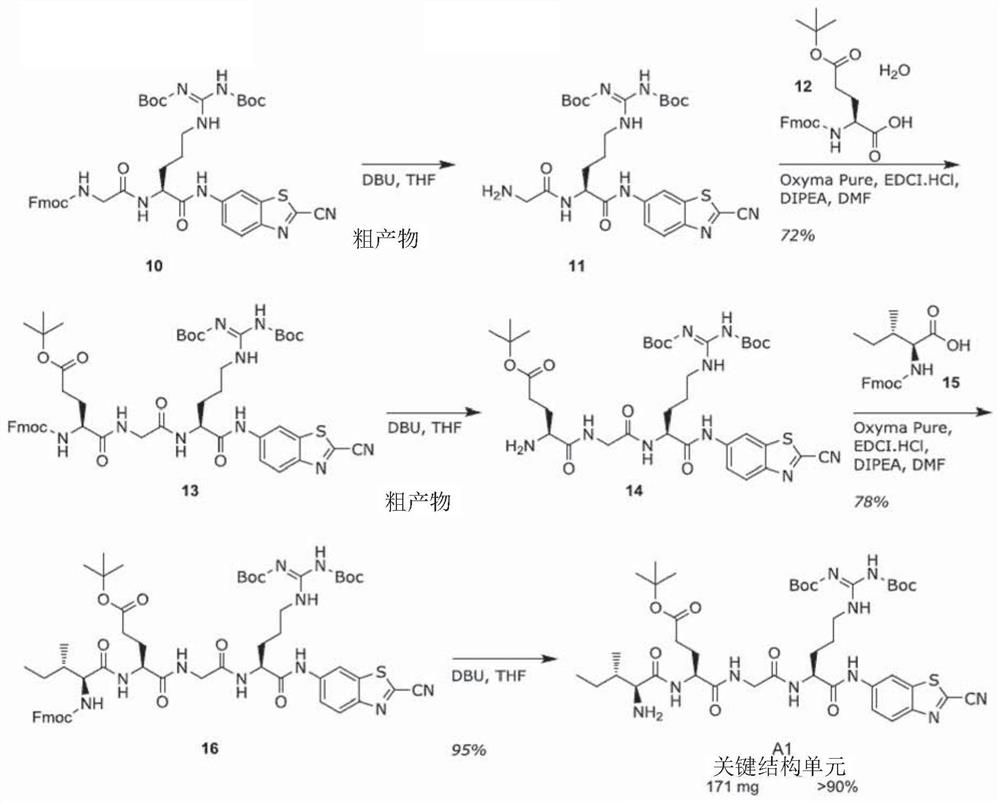
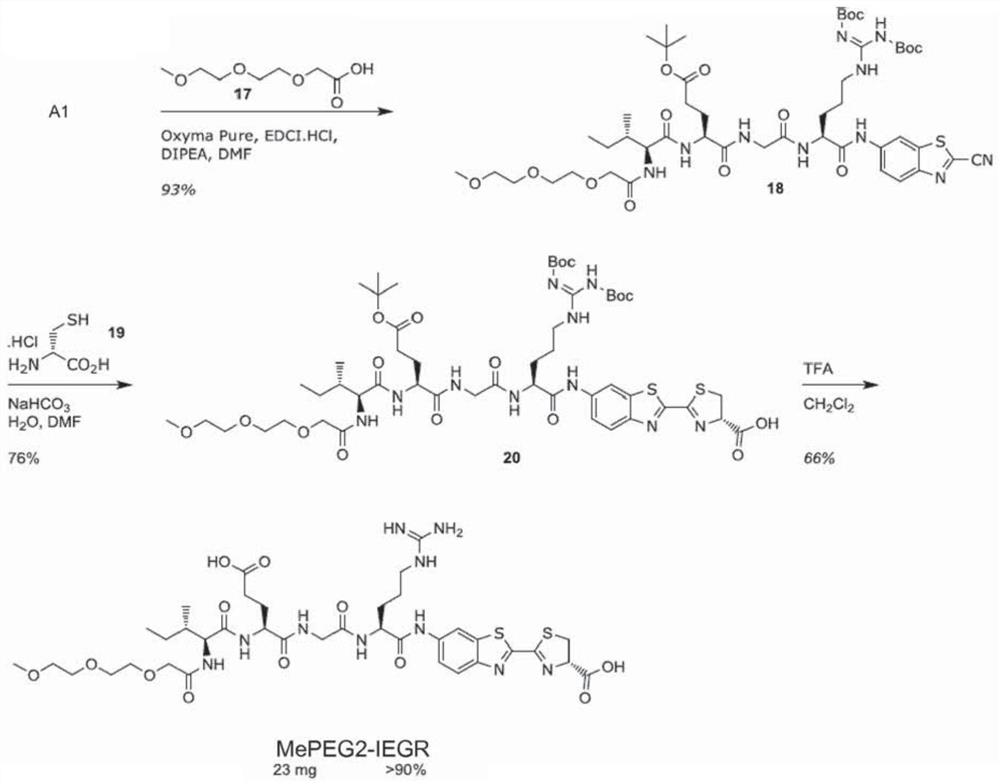
[0181] Such as Figure 1A As shown, the substrate is synthesized through the key intermediate A1. The synthesis of this intermediate started with the conversion of benzothiazole 1 to nitrile 2 in 91% yield. Reduction afforded pure aniline in 54% yield after direct and reverse phase flash column chromatography. In pyridine, with PCl 3 Coupling with Fmoc-Orn(Boc)-OH(4). After Fmoc-deprotection, compound 6 was successfully obtained. In these two steps, peptide coupling of 6 with Fmoc-Gly-OH 7 to 8 and Boc deprotection gave 9 in 84% yield. After purification by flash column chromatography, reaction with 1,3-bis(tert-butoxycarbonyl)-2-(trifluoromethanesulfonyl)guanidine afforded guanidine 10 in good yield and purity. To provide compounds of general formula 1-3 or 1-4 in which other amino acids are present, the above scheme is repeated with said other amino acids, or compound 8 is not converted to compound 10.
[018...
Embodiment 2
[0184] Example 2 - Kinetic Behavior of Substrates of the Invention
[0185] The Michaelis-Menten kinetics of the enzyme FXa and its substrate MePEG2-IEGR were determined (TFA salt was used in these examples). Prepare the following compositions in microtubes on ice:
[0186] Composition 1:
[0187] 160 μL Tris-buffered saline (TBS) 50 mM Tris-HCl, 150 mM NaCl (pH 7.4)
[0188] 4 μL ATP (final concentration 333 μM)
[0189] 4 μL MgCl 2 (final concentration 8.3mM)
[0190] 12 μL luciferase (final concentration 0.9 mg / ml)
[0191] Substrate compositions 2a-g:
[0192] Prepare microtiter tubes with 30 µL of substrate concentrations resulting in final concentrations of 2000, 1333, 1000, 666, 333, 167, and 66 µM.
[0193] Enzyme composition 3:
[0194] Prepare FXa (Coachrom, 16nM) compositions in microtubes on ice
[0195] Subsequently, the following compositions were added together to a white 384-well plate:
[0196] 24 μL composition 1
[0197] 3 μL of compositions 2a-g ...
Embodiment 3
[0201] Example 3 - Cross-reactivity
[0202] To determine cross-reactivity, assays were performed using a series of coagulases at concentrations representative of those found in human plasma. The selected enzyme concentrations were comparable to those expected in vivo after activation. For tissue plasminogen activator (tPA), this is the usual concentration to promote fibrinolysis.
[0203] FXa at a concentration of 80 nm produced a signal of 1.000.000 RLU. The other thrombins produced the following signals: 52 nM thrombin -30,000 RLU, 8 nM plasmin -8,000 RLU, 193 IU / mL tPA -6,000 RLU, 145 nMFXIIa -500 RLU (Figure 3). These data indicate that the substrates of the invention are preferentially cleaved by FXa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com