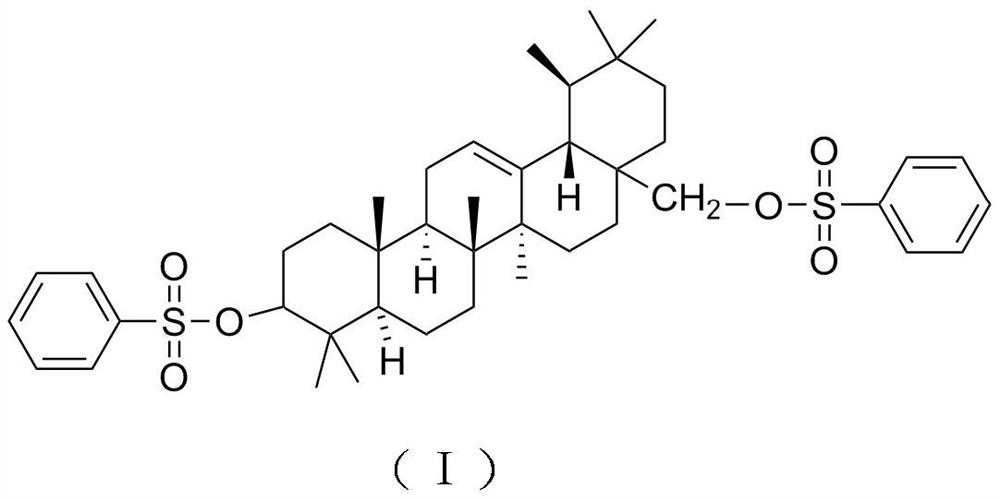
Oleanolic acid derivative for treating psoriasis and preparation method thereof
A technology of oleanolic acid and derivatives, which is applied in the field of chemical drug synthesis, and can solve the problems of human body irritating response and limited bioavailability application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
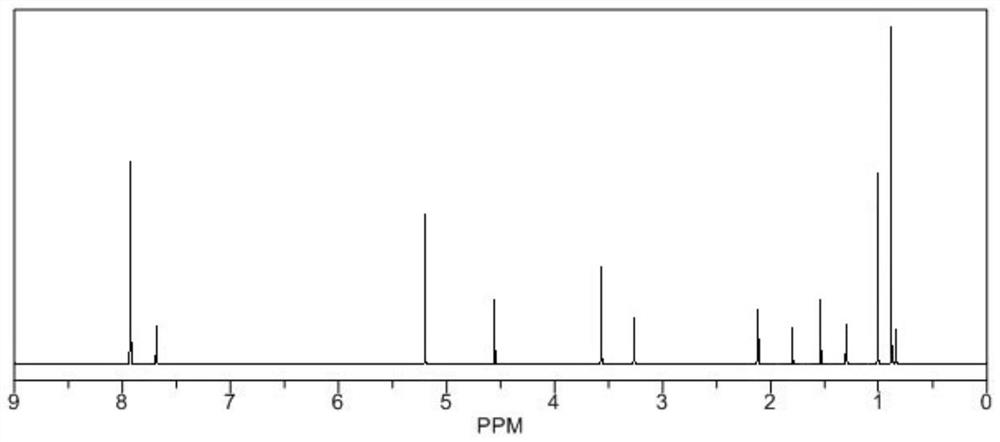
[0024] (1) Dissolve 15g of oleanolic acid in 100mL of tetrahydrofuran, add 3.7g of borane dimethyl sulfide complex, reflux at a temperature of 0-5°C for 13-15 hours, raise the temperature to room temperature, filter to remove the precipitate, and concentrate the filtrate into an extract, rinse with water, then rinse with 10% ethanol for 2 to 8 BV, then rinse with 80% ethanol, and dry in vacuum;
[0025] (2) Dissolve the product of step (1) in 100 mL of dichloromethane, add benzenesulfonyl chloride, add sodium hydroxide, reflux at room temperature for 20-24 hours, filter, concentrate to remove the organic solvent, add saturated sodium bicarbonate solution, and use Petroleum ether was extracted 2 to 3 times, the petroleum ether layers were combined, dried with anhydrous magnesium sulfate, concentrated, and the concentrated product was subjected to silica gel column chromatography, and the mixture of ethyl acetate and acetone with a volume ratio of 10:1 was used as the eluent Gra...
experiment example 2
[0026] Experimental Example 2 Pharmaceutical Research of Oleanolic Acid Derivatives on Psoriasis
[0027] 1. Experimental method
[0028] Healthy mice of the Kunming species were selected, cultured in a suitable environment, anesthetized by intraperitoneal injection of 1% chloral hydrate (0.2mL / 20g), and the hair on the back of the mice was removed to form a naked area with a size of 2cm×3cm. The mice were randomly divided into: normal group (control group 1), model group (control group 2), halometasone group (control group 3), oleanolic acid group (control group 4), oleanolic acid derivatives group (experimental group). Normal group: 42 mg of Vaseline ointment was applied to the exposed parts of the mice every day; model group: 42 mg of imiquimod was applied to the exposed areas of the back of the mice once a day for 7 consecutive days; Halometasone group: the exposed parts of the mice were regularly treated in the morning Apply imiquimod 42mg / time, once a day, and halometa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com