Tolvaptan oral preparation and preparation method thereof
A technology of tolvaptan and oral preparations, applied in the field of chemical pharmaceuticals, can solve the problems of affecting bioavailability, dissolution rate reduction, etc., and achieve the effect of ensuring bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
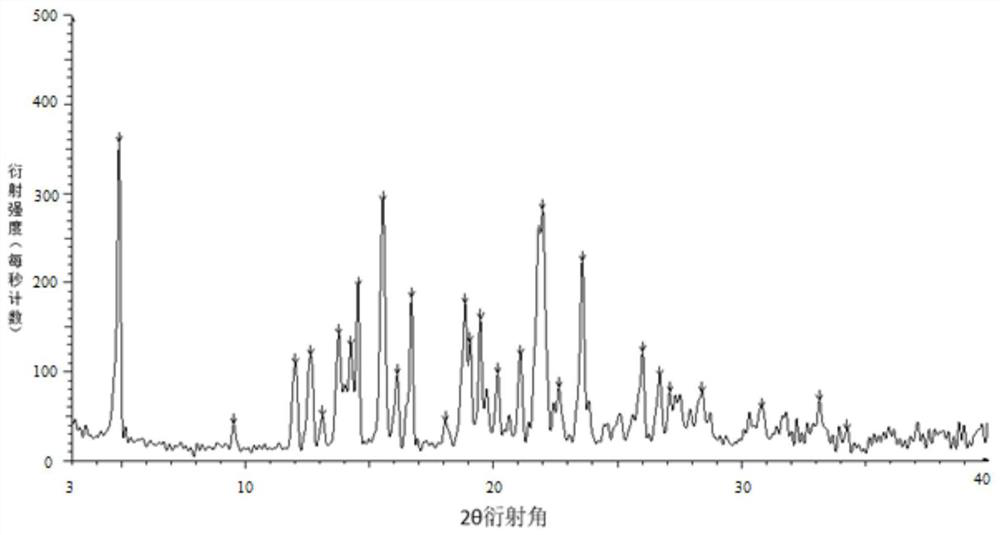
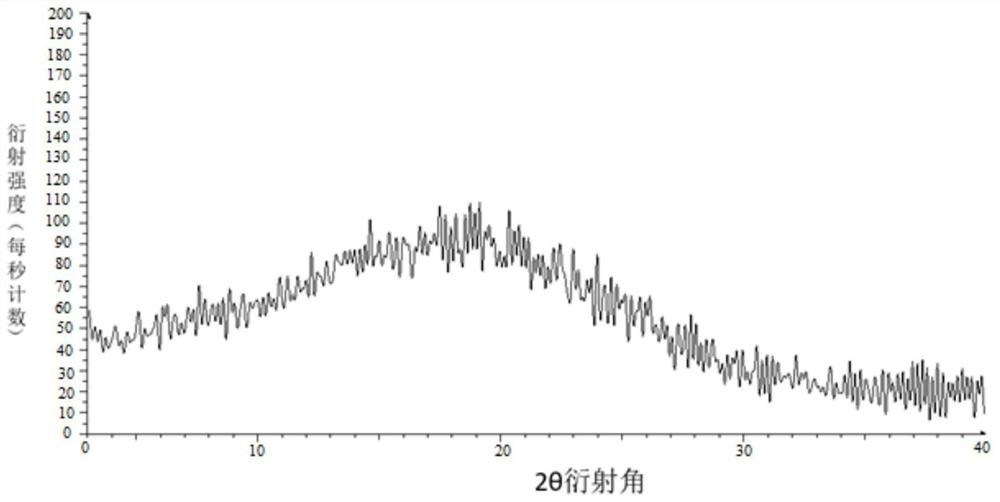
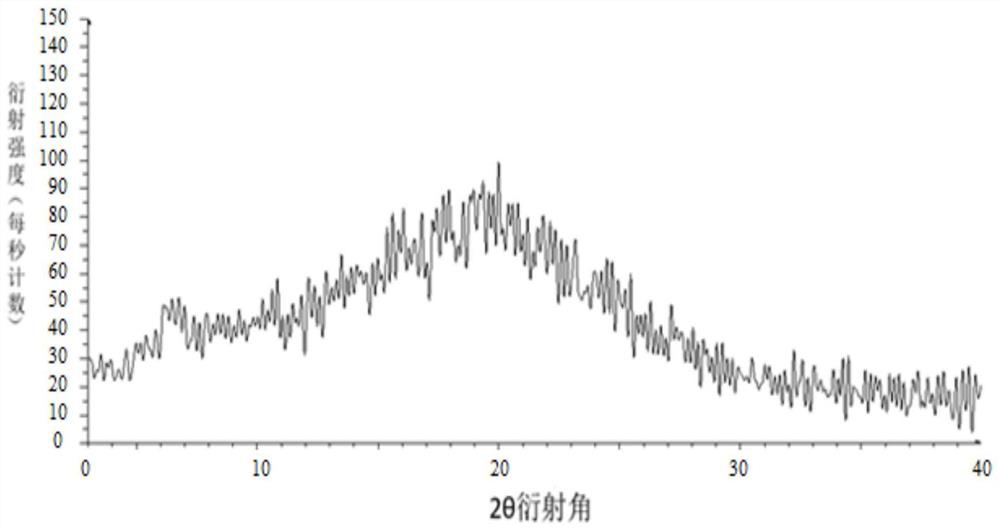
Image
Examples
Embodiment 1
[0045] Weigh 100 g of tolvaptan bulk drug and 50 g of hydroxypropyl cellulose, add them into 350 g of absolute ethanol and 1150 g of dichloromethane mixed solvent, and stir to dissolve. Use an explosion-proof closed-type nitrogen circulation spray dryer for spray drying, set the inlet air temperature to 100°C; the rotation speed of the atomization disc is 30,000rpm, the liquid supply speed is 5kg / h, and the outlet air temperature is 40-70°C, pump the mixed solution with a peristaltic pump Put it into a spray dryer, dry under reduced pressure at 40°C for 12 hours, remove organic reagents, and pass through a 40-mesh sieve to obtain a water-soluble solid dispersion.
Embodiment 2
[0047] Weigh 100 g of tolvaptan bulk drug and 100 g of hypromellose phthalate, add them to 900 g of absolute ethanol and 1650 g of dichloromethane mixed solvent, and stir to dissolve. Use an explosion-proof closed-type nitrogen circulation spray dryer for spray drying, set the inlet air temperature to 100°C; the rotation speed of the atomization disc is 30,000rpm, the liquid supply speed is 5kg / h, and the outlet air temperature is 40-70°C, pump the mixed solution with a peristaltic pump Put it into a spray dryer, dry under reduced pressure at 40°C for 12 hours, remove organic reagents, and pass through a 40-mesh sieve to obtain an enteric solid dispersion.
Embodiment 3
[0049] Weigh 100 g of tolvaptan bulk drug and 50 g of hydroxypropyl cellulose, add them into 700 g of absolute ethanol and 2300 g of dichloromethane mixed solvent, and stir to dissolve. Use an explosion-proof closed-type nitrogen circulation spray dryer for spray drying, set the inlet air temperature at 140°C; the rotation speed of the atomization disc is 30,000rpm, the liquid supply speed is 10kg / h, and the outlet air temperature is 80-110°C, pump the mixed solution with a peristaltic pump Put it into a spray dryer, dry under reduced pressure at 40°C for 12 hours, remove organic reagents, and pass through a 40-mesh sieve to obtain a water-soluble solid dispersion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com