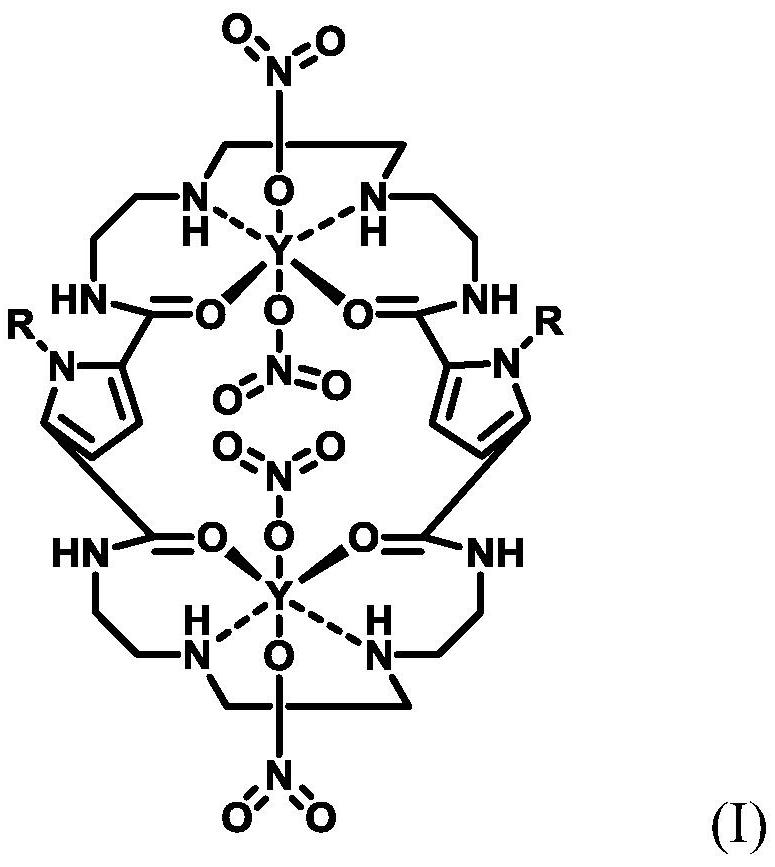
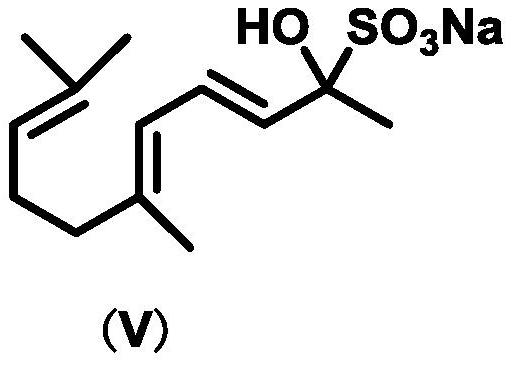
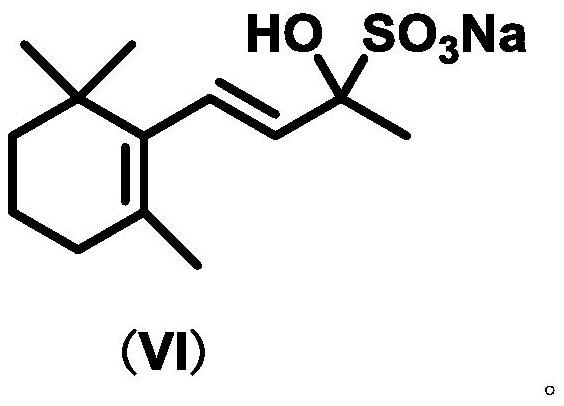
Hydrophobic catalyst, preparation method thereof and preparation method of beta-ionone
A technology of ionone and pseudoionone, applied in chemical instruments and methods, oxidation preparation of carbonyl compounds, physical/chemical process catalysts, etc., can solve problems such as low economic benefit, high cost of sulfuric acid wastewater treatment, and easy clogging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] To prepare the hydrophobic catalyst H-C1, proceed as follows:
[0079] (1) Dissolve N-methylpyrrole (40.56g, 0.5mol) in ether (162.23g), mix well and prepare solution a; dissolve trichloroacetyl chloride (186.37g, 1.03mol) in ether (1677.30 In g), mix evenly to prepare solution b; under nitrogen protection and stirring, at a reaction temperature of 40°C, add solution a dropwise to solution b for 7 hours, and continue to Stir the reaction for 4h, then quench the reaction with potassium carbonate aqueous solution (5wt%, 921.40g), separate the ether phase after standing, remove the ether in vacuum, recrystallize with isopropanol, and obtain a solid after vacuum drying Its nuclear magnetic analysis data are: 1 H NMR (400MHz, CDCl 3 ,298K)δ=7.50(2H,s, Ar- H ),3.59(3H,s,N CH 3 ).
[0080] (2) Will (74.37g, 0.2mol) was dissolved in N, N-dimethylformamide (855.23g), mixed uniformly and prepared into solution c; triethylenetetramine (30.71g, 0.21mol) was dissolved in ...
Embodiment 2
[0083] To prepare the hydrophobic catalyst H-C2, proceed as follows:
[0084] (1) Dissolve N-methylpyrrole (40.56g, 0.5mol) in ether (60.84g), mix well and prepare solution a; dissolve trichloroacetyl chloride (272.73g, 1.50mol) in ether (636.37 In g), mix evenly to prepare solution b; under nitrogen protection and stirring, at a reaction temperature of 80°C, add solution a dropwise to solution b for 3 hours, and continue to Stir the reaction for 2h, then quench the reaction with potassium carbonate aqueous solution (20wt%, 138.21g), separate the ether phase after standing, remove the ether in vacuum, recrystallize with isopropanol, and obtain a solid after vacuum drying
[0085] (2) Will (74.37g, 0.2mol) was dissolved in N, N-dimethylformamide (338.79g), mixed uniformly and prepared into solution c; triethylenetetramine (35.10g, 0.24mol) was dissolved in N, N - Dimethylformamide (184.26g), mixed evenly to prepare solution d; under nitrogen protection and stirring, at a r...
Embodiment 3
[0088] Prepare hydrophobic catalyst H-C3, carry out according to the following steps:
[0089] (1) Dissolve N-ethylpyrrole (47.57g, 0.5mol) in ether (111.00g), mix well and prepare solution a; dissolve trichloroacetyl chloride (227.28g, 1.25mol) in ether (909.11 In g), mix uniformly to prepare solution b; under nitrogen protection and stirring, at a reaction temperature of 60°C, add solution a dropwise to solution b for 5 hours, and continue to Stir the reaction for 3h, then quench the reaction with potassium carbonate aqueous solution (12.5wt%, 276.42g), separate the ether phase after standing, remove the ether in vacuum, recrystallize with isopropanol, and obtain a solid after vacuum drying
[0090] (2) Will (77.17g, 0.2mol) was dissolved in N, N-dimethylformamide (516.47g), mixed uniformly and prepared into solution c; triethylenetetramine (33.05g, 0.23mol) was dissolved in N, N -Dimethylformamide (221.18g), mixed evenly to prepare solution d; under nitrogen protection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com