N-acyl homoserine lactone acyltransferase coding gene aigC and application thereof
A technology of acyl homoserine and lactone acyl, applied in the fields of application, genetic engineering, plant genetic improvement, etc., can solve problems such as microbial resistance, pesticide and antibiotic environmental safety, and human and animal health threats, so as to weaken pathogenicity, Impaired motility, broad-spectrum, and highly efficient activity-quenching effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Acquisition and Identification of AHLs Degradation Genes
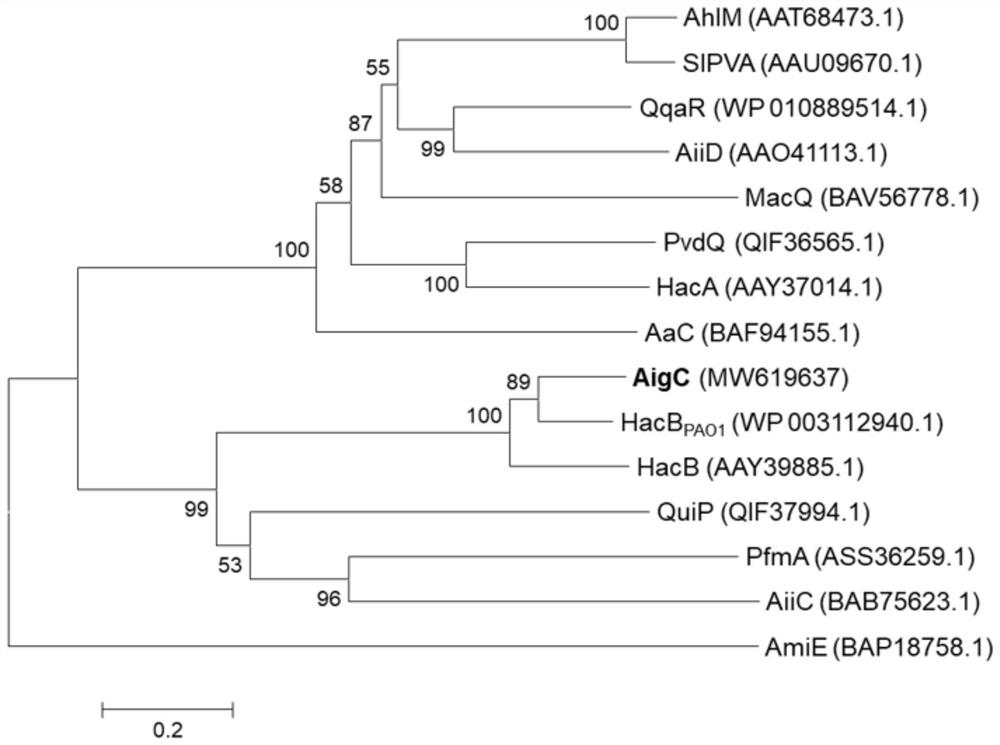
[0054] According to the whole genome sequencing results of Pseudomonas nitroreductor strain HS-18 in the previous work, the gene aigC that may encode N-acyl homoserine lactone acyltransferase was found by using genome annotation and bioinformatics comparison. The amino acid sequences of AigC and the currently known N-acyl homoserine lactone acyltransferases were compared using the software MEGA 5.10, and the phylogenetic evolution analysis was carried out and the evolutionary tree was constructed using ClustalX1.8.3 and the neighbor-joining method. The result is as figure 2 showed that AigC interacts with the known AHLs acylase HacB in Pseudomonas aeruginosa PAO1 PAO1 High amino acid similarity (76.6%).
[0055] Design primers to amplify and construct primers for inserting aigC into the broad host vector pBBR1 and protein prokaryotic expression vector pET32a recombinant vector respectively. The prim...
Embodiment 2
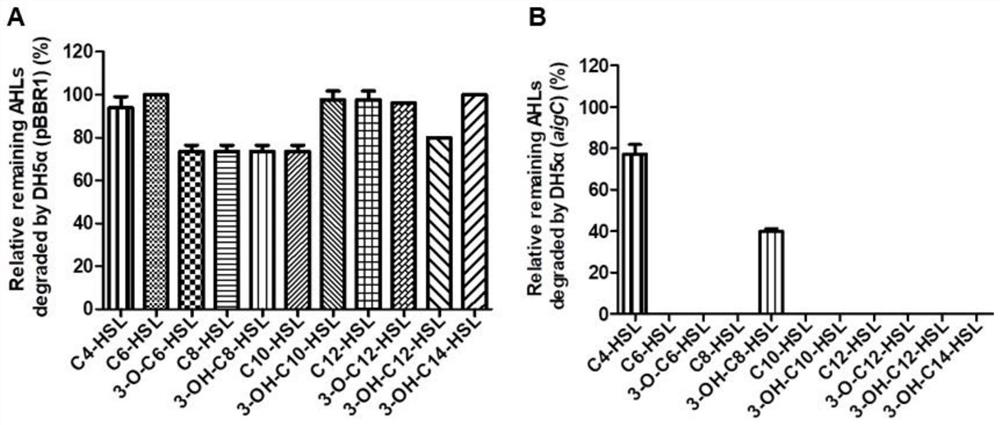
[0060] Example 2 Detection of recombinant bacteria DH5α (aigC) on AHLs quenching activity
[0061] AHLs degradation system setup: Cultivate successfully constructed DH5α(aigC) overnight in LB liquid medium to obtain OD 600 =1.0 seed liquid, adding equal volume of fresh LB liquid medium and exogenous final concentration of 10-50 μM of different carbon chain lengths and different substituents of AHLs and final concentration of 50mM MOPS to prepare the degradation system (different AHLs signal Select the appropriate concentration according to the different intensity of color development of the reporter strain), and cultivate it in a constant temperature shaker at 37°C and 200rpm for 36 hours, using DH5α (pBBR1) bacterial liquid and LB liquid medium without bacterial liquid as controls. Secondly, extract the culture solution with an equal volume of ethyl acetate, and use the reporter strain to detect the remaining AHLs content in 10 μl of the ethyl acetate extract.
[0062] Quant...
Embodiment 3
[0064] Example 3 Prokaryotic expression of AigC protein
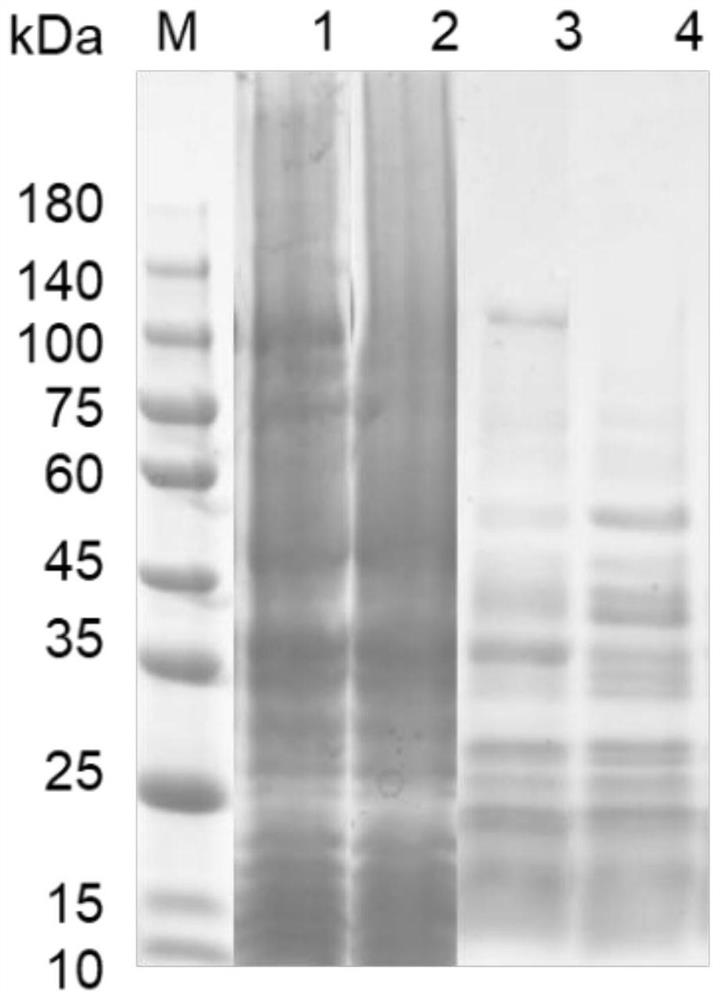
[0065] The successfully constructed recombinant strain BL21(DE3)(pET32a-aigC) was cultured overnight in LB liquid medium supplemented with a final concentration of 100 μg / ml ampicillin, and cultivated in a constant temperature shaker at 37°C at 200 rpm to obtain seed liquid. Then add the seed liquid at a ratio of 1:100 to fresh LB liquid medium containing ampicillin with a final concentration of 100 μg / ml, and cultivate it in a constant temperature shaker at 37°C and 200 rpm until OD 600 = 0.6-0.8, adding 0.5mM IPTG at a final concentration for induction, and culturing overnight at 18°C and 200rpm in a constant temperature shaker. The cells cultured overnight were collected, and the cells were disrupted, and the expression of AigC was identified by SDS-PAGE electrophoresis. according to image 3 The results showed that AigC carrying His tag could be expressed in normal prokaryotic cells, and the size was about 106.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com