Coordination compound phosphorescent material based on electron-deficient functional group
A technology of functional groups and phosphorescent light-emitting materials, applied in the field of complex phosphorescent materials, can solve the problem of not paying much attention to the excited state of higher energy level, and achieve optimized carrier injection and transport properties, excellent display effect, and enhanced electroluminescence. effect of ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
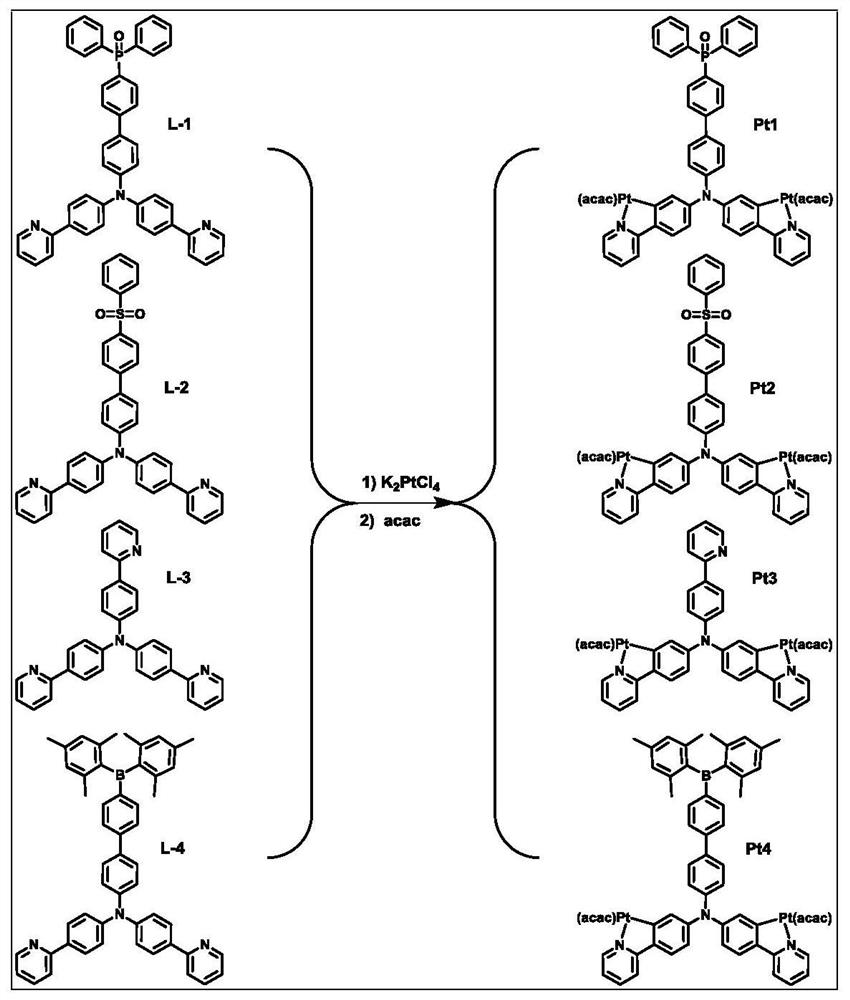
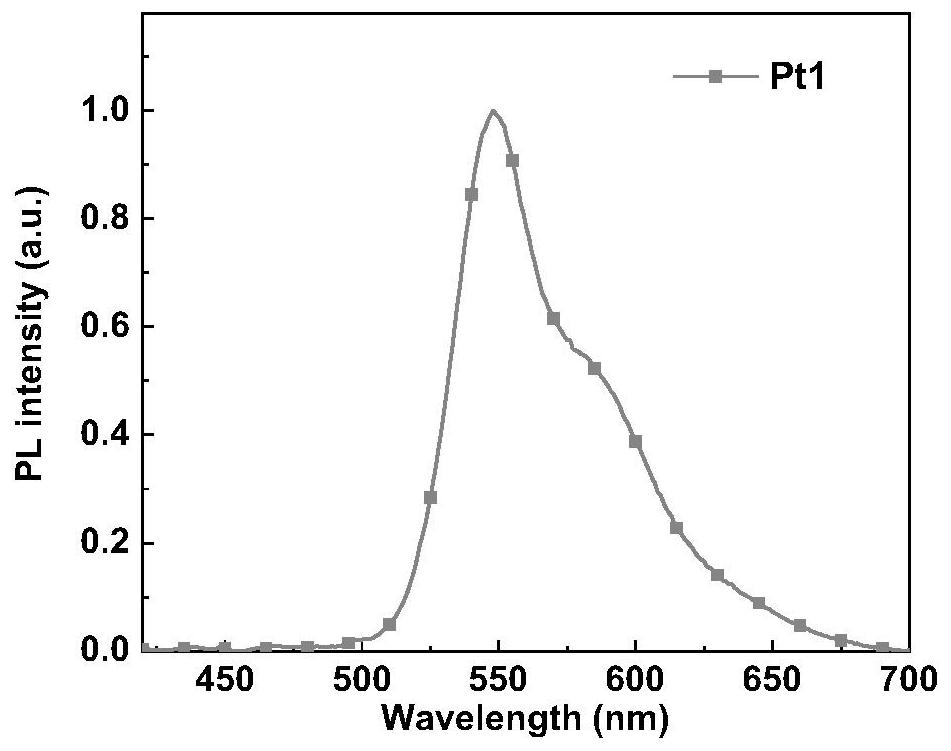
[0034] The structural formula of ligand L-1 based on the electron-deficient functional group of this embodiment is refer to figure 1 , its synthesis method is: 1 equivalent electron-deficient functional group compound and 1 equivalent of the tetradentate precursor Put into reaction vessel, under nitrogen atmosphere, add 0.05% equivalent tetrakis(triphenylphosphine palladium) catalyst, 50 milliliters of tetrahydrofuran and 30 milliliters of concentrations in said reaction vessel and be the potassium carbonate solution of 2mol / L, under nitrogen atmosphere Heat to 110° C. to react overnight; stop heating, cool to room temperature, add deionized water to the reaction mixture solution, and extract the reaction mixture with dichloromethane. The obtained organic phase was dried with anhydrous sodium sulfate, concentrated, and separated and purified on a silica gel column to obtain the corresponding ligand L-1 based on an electron-deficient functional group. The NMR characteriza...
Embodiment 2
[0037] The structural formula of ligand L-2 based on the electron-deficient functional group of this embodiment is refer to figure 1 , its synthesis method is: 1 equivalent electron-deficient functional group compound and 1 equivalent of the tetradentate precursor Put into the reaction vessel, under nitrogen atmosphere, add 0.05% equivalent tetrakis(triphenylphosphine palladium) catalyst, 50 milliliters of tetrahydrofuran and 30 milliliters of concentration in the reaction vessel and be the potassium carbonate solution of 2mol / L, under nitrogen atmosphere Heat to 110°C to react overnight; stop heating, add deionized water to the reaction mixture solution after cooling to room temperature, and extract the reaction mixture with dichloromethane; the obtained organic phase is dried with anhydrous sodium sulfate, concentrated, and separated and purified by silica gel column, namely The corresponding ligand L-2 based on electron-deficient functional groups can be obtained. The...
Embodiment 3
[0040] The structural formula of ligand L-3 based on the electron-deficient functional group of this embodiment is refer to figure 1 , its synthesis method is: 1 equivalent electron-deficient functional group compound and 1 equivalent of the tetradentate precursor Put into reaction vessel, under nitrogen atmosphere, add 0.05% equivalent tetrakis(triphenylphosphine palladium) catalyst, 50 milliliters of tetrahydrofuran and 30 milliliters of concentrations in said reaction vessel and be the potassium carbonate solution of 2mol / L, under nitrogen atmosphere Heat to 110°C and react overnight; stop heating, add deionized water to the reaction mixture solution after cooling to room temperature, and extract the reaction mixture with dichloromethane; the obtained organic phase is dried with anhydrous sodium sulfate, concentrated, and separated and purified by silica gel column, namely The corresponding ligand L-3 based on electron-deficient functional groups can be obtained. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com