Dithiophospholipid compound and preparation method thereof
A technology of dithiophospholipids and compounds, applied in the direction of phosphorus organic compounds, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc., can solve problems such as limited application range, lack of GSH response fracture, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
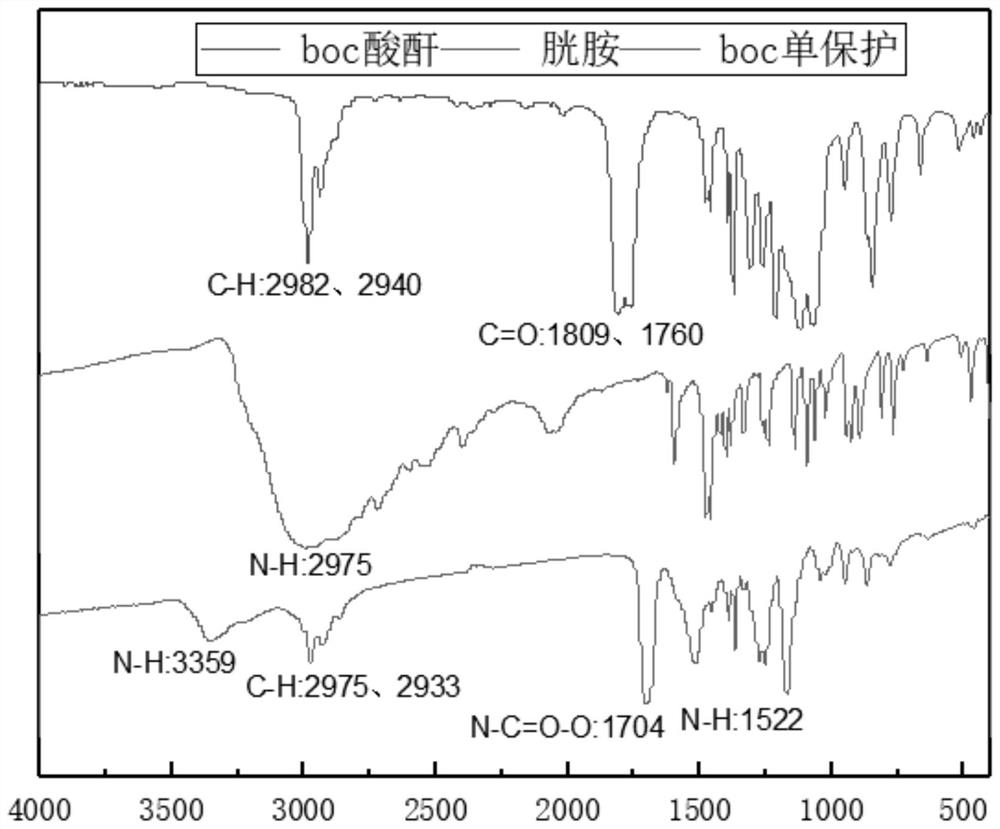
[0042] 1) Dissolve about 10 mmol of cystamine hydrochloride in anhydrous methanol, add 20 mmol of triethylamine, dissolve 15 mmol of di-tert-butyl dicarbonate in a small amount of dichloromethane, add dropwise to the above methanol solution, and react at 20°C for 10 hours. After the reaction was completed, 1.7 g of Boc mono-protected cystamine was obtained by separation and purification. The infrared spectrum of monoprotected cystamine is shown in figure 1 .
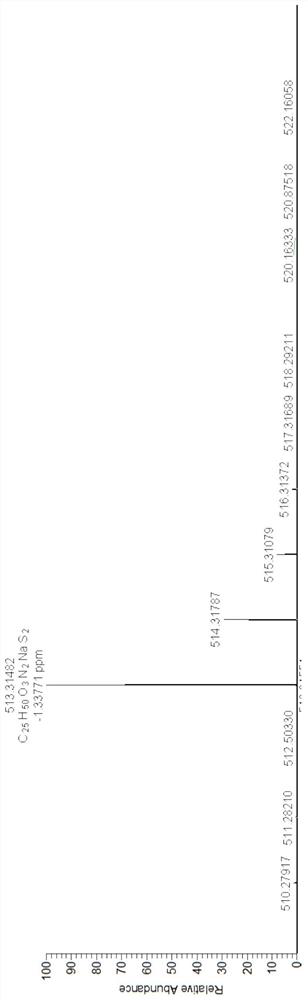
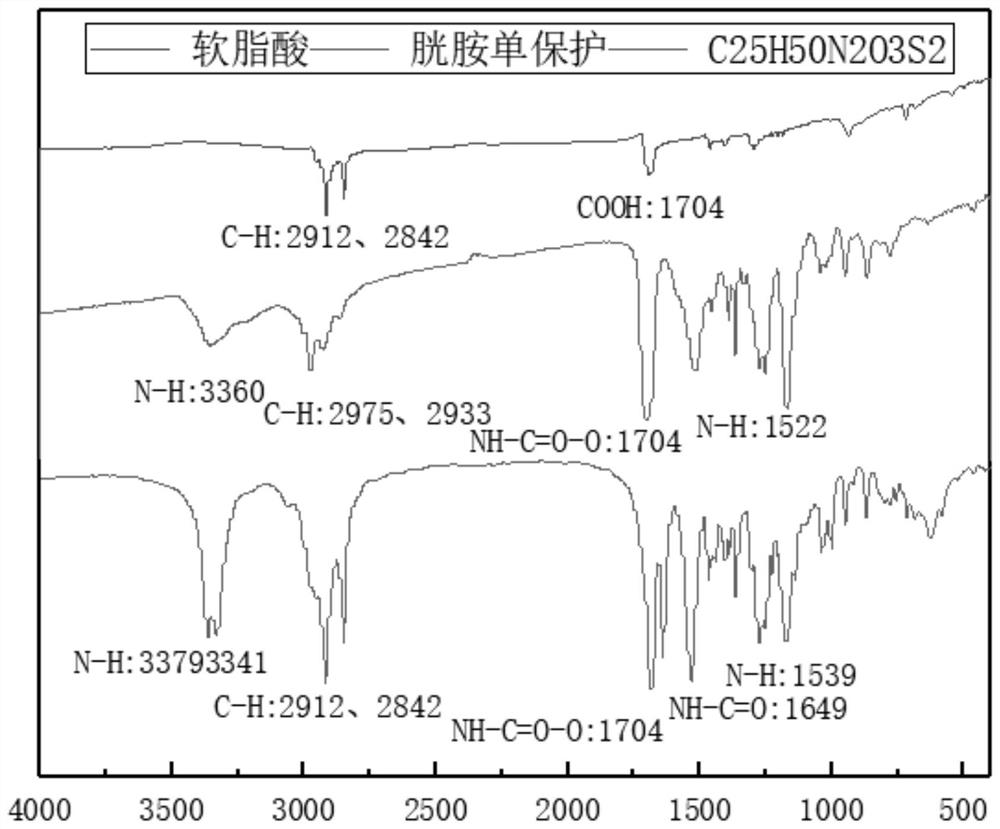
[0043] 2) Dissolve 15mmol palmitic acid in dichloromethane, add 20mmol 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 12mmol N-hydroxysulfosuccinimide, room temperature After activation, add 10 mmol monoprotected cystamine and react at 20°C for 10 h. After the reaction finished, the solid 10mmol obtained by separation and purification (see figure 2 ) was dissolved in dichloromethane, 20% trifluoroacetic acid was added, reacted at 20° C. for 12 hours, separated and purified to obtain 1.5 g of light yello...
Embodiment 2
[0047] 1) Dissolve about 15 mmol of cystamine hydrochloride in anhydrous methanol, add 20 mmol of triethylamine, dissolve 10 mmol of di-tert-butyl dicarbonate in a small amount of dichloromethane, add dropwise to the above methanol solution, and react at 25°C for 12 hours. After the reaction was completed, 1.8 g of Boc mono-protected cystamine was obtained by separation and purification.
[0048] 2) Dissolve 10mmol stearic acid in dichloromethane, add 12mmol 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 15mmol N-hydroxysulfosuccinimide, room temperature Activated under low temperature, add 12mmol mono-protected cystamine, and react at 25°C for 10h. After the reaction, the product was obtained by separation and purification.
[0049] 3) Dissolve 10 mmol of the product in the previous step with dichloromethane, add 15% trifluoroacetic acid, react at 25° C. for 12 h, and separate and purify the product after the reaction ends.
[0050] 4) Dissolve 10 mmol of the ...
Embodiment 3
[0053] 1) Dissolve about 10 mmol of cystamine hydrochloride in anhydrous methanol, add 30 mmol of triethylamine, dissolve 15 mmol of di-tert-butyl dicarbonate in a small amount of dichloromethane, add dropwise to the above methanol solution, and react at 30°C for 12 hours. After the reaction, 1.9 g of Boc mono-protected cystamine was obtained by separation and purification.
[0054] 2) Dissolve 10mmol myristic acid in dichloromethane, add 20mmol 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 15mmol N-hydroxysulfosuccinimide, at room temperature For activation, add 10 mmol monoprotected cystamine and react at room temperature for 15 h. After the reaction was completed, 2.3 g of a light yellow solid was obtained by separation and purification.
[0055] 3) Dissolve 10 mmol of the product in the previous step with dichloromethane, add 15% trifluoroacetic acid, stir at room temperature for 8 h, and separate and purify to obtain 1.5 g of a light yellow solid after the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com