Preparation method of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and hydroxychloroquine, applied in the field of medicine and chemical industry, can solve the problems of a large amount of solvent extraction or washing, the large amount of solvent is not environmentally friendly, and the purity of the finished product is not satisfactory.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0096] The preparation method of hydroxychloroquine sulfate
[0097] The object of the present invention is to provide a new production process of hydroxychloroquine sulfate or a preparation method of hydroxychloroquine sulfate with short reaction time, environmentally friendly solvent, simple operation and controllable quality.
[0098] Typically, the preparation method provided by the invention comprises steps:
[0099]
[0100] (1) the step of preparing hydroxychloroquine, comprising steps:
[0101] (1.1) 4,7-dichloroquinoline (formula I), amino side chain (formula II), potassium iodide and antioxidant (preferably, antioxidant is one or more in BHA, BHT; More preferably (for example, BHA) is added to the first solvent (for example, n-pentanol), and the temperature is raised to a certain temperature (such as 137~147° C.) under the protection of an inert gas to react, thereby obtaining the reaction containing hydroxychloroquine (formula III) mixture;
[0102] (1.2) Remo...
Embodiment 11
[0152] The preparation of embodiment 1.1 hydroxychloroquine
[0153] The preparation of hydroxychloroquine crude product:
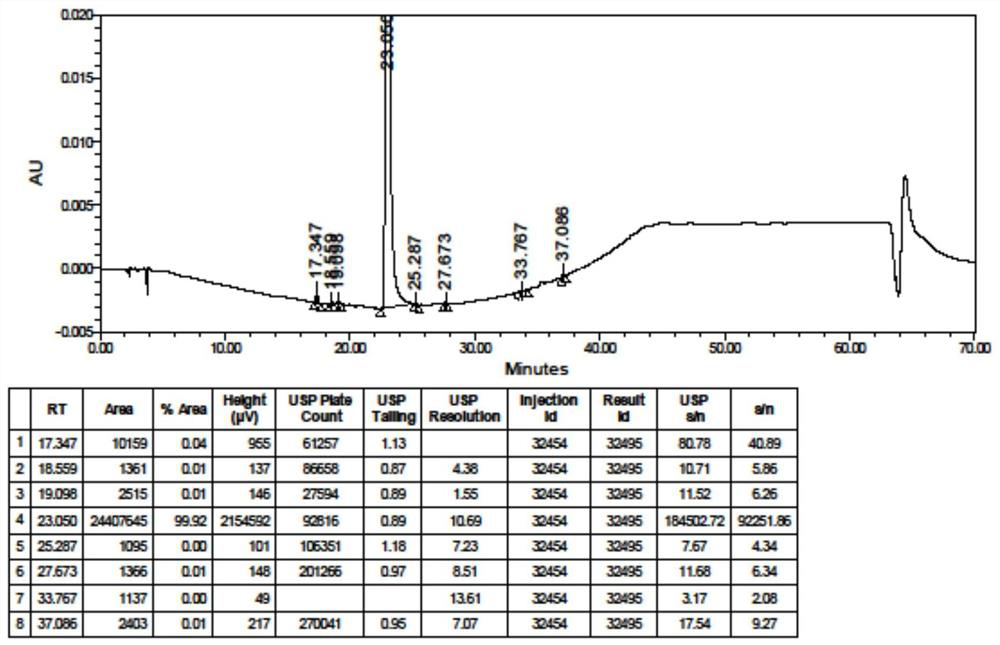
[0154] 100.01g (0.50mol) of 4,7-dichloroquinoline, 105.68g (0.61mol) of aminoamylaminoalcohol (ie amino side chain), 0.858g (0.005mol) of potassium iodide, and 4.54 g (0.025mol) of BHA and n-pentanol 200mL were added to the reactor, replaced with nitrogen 3 times and protected by nitrogen, heated to reflux (137-147°C) and reacted for 15 hours. The temperature was lowered to below 90°C, and the solvent was evaporated under reduced pressure. Cool down to below 70°C, add dilute hydrochloric acid (5-6% dilute hydrochloric acid) to adjust the pH to 1-3, wash the water phase with 200mL x 3 ethyl acetate, then add 500mL of dichloromethane, stir and cool down to below 20°C, slowly Add 40% sodium hydroxide aqueous solution, adjust the pH to about 12, separate the layers, extract the aqueous phase with dichloromethane twice (500 mL, 200 mL), and combine the o...
Embodiment 12
[0159] The preparation of embodiment 1.2 hydroxychloroquine sulfate
[0160] The preparation of hydroxychloroquine sulfate crude product:
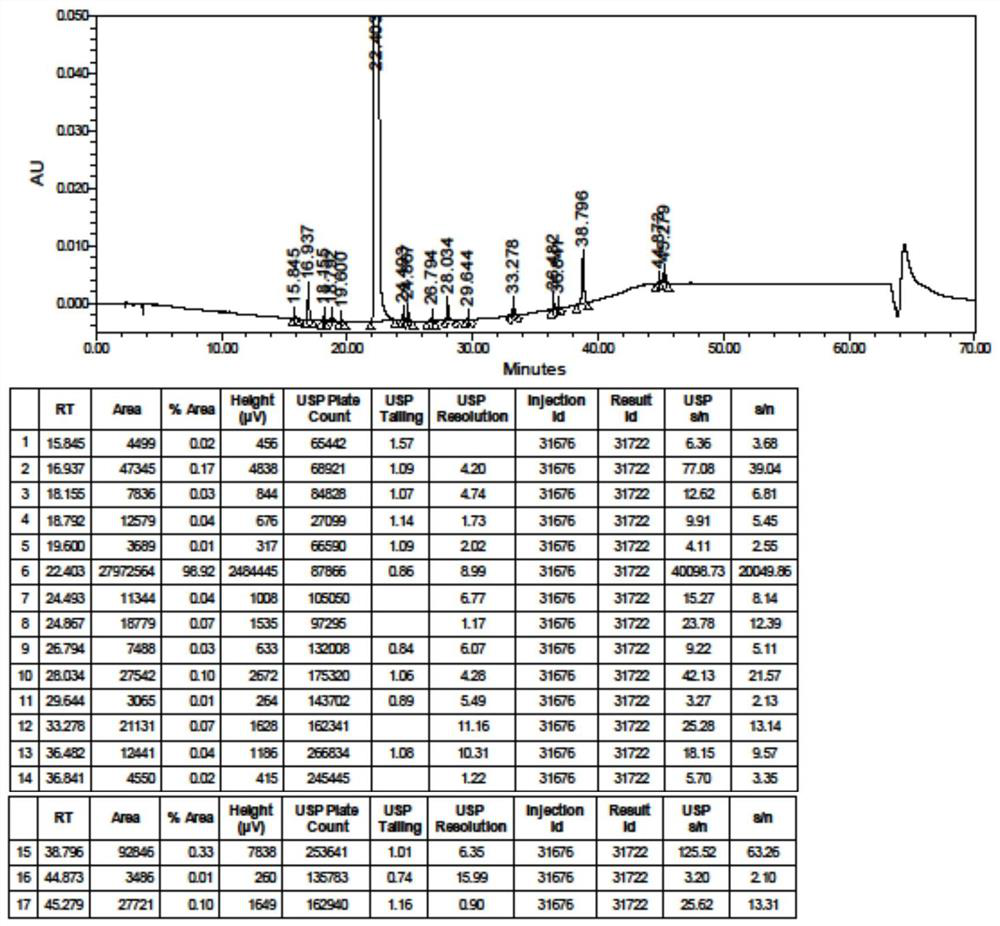
[0161] Put 84.88g (0.25mol) of refined hydroxychloroquine (0.25mol) and 915mL of 95% ethanol into the reactor, stir and dissolve, cool the reaction mass to 0-10°C, add 24.07g of concentrated sulfuric acid dropwise, continue stirring for 0.5 hours after the dropwise addition, and then raise the temperature Stir at 20-30°C, filter, rinse the filter cake with 95% ethanol 100mL x 3, and dry the wet product in vacuum at 80°C for 22 hours to obtain 101.63g (0.23mol) of crude hydroxychloroquine sulfate, HPLC purity 99.87%, yield 92.7 %.
[0162] The preparation of hydroxychloroquine sulfate finished product:
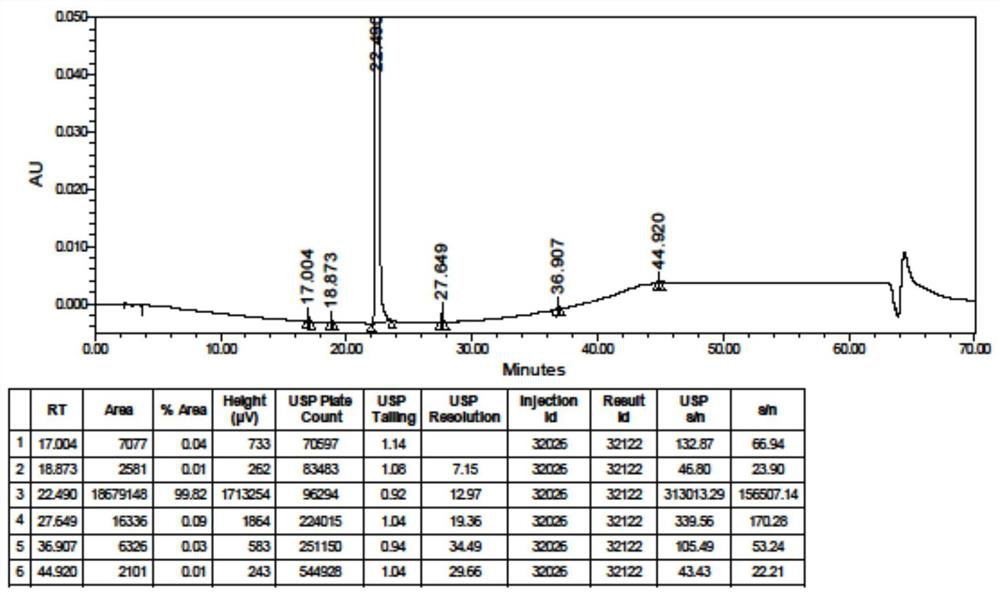
[0163] Put 101.5g (0.23mol) of hydroxychloroquine sulfate crude product, 50.5mL of purified water, 152mL of absolute ethanol and 5.07g of activated carbon into the reactor, heat to reflux, stir at reflux for 0.5 hours, filter while ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com