Application of 4-aminoquinoline compound in treatment of coronavirus infection
A composition, hydrate technology, applied in the field of treatment of SARS-CoV-2 infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: Chloroquine phosphate reduces the cell viral nucleic acid load experiment of SARS-CoV-2 infection
[0069] (1) Drug treatment of virus-infected cells
[0070] Inoculate Vero E6 cells (purchased from ATCC, Cat. No. 1586) into 24-well plates and culture for 24 hours; then carry out virus infection, specifically, with 2% cell maintenance solution (formulation: FBS (purchased from Gibco, Cat. No. 16000044) Add MEM (purchased from Gibco, product number 10370021) according to the volume ratio of 2%, which is 2% cell maintenance solution) SARS-CoV-2 (2019-nCoV) virus (nCoV-2019BetaCoV / Wuhan / WIV04 / 2019 strain, Stored by the Wuhan Institute of Virology, Chinese Academy of Sciences) diluted to the corresponding concentration, and then added to a 24-well plate so that each well contains 100 TCID of virus 50 . Next, dilute chloroquine phosphate and hydroxychloroquine (purchased from Sigma-Aldrich Company, product number C6628 with 2% cell maintenance solution; In t...
Embodiment 2
[0106] Embodiment 2: Chloroquine phosphate and hydroxychloroquine reduce the cellular viral nucleic acid load experiment of SARS-CoV-2 infection under 4 kinds of different infection titers (MOI)
[0107] (1) Drug treatment
[0108]Vero E6 cells were inoculated into 24-well plates, cultured for 24 hours, and then virus-infected. Set four different infection doses, respectively 0.01MOI, 0.02MOI, 0.2MOI and 0.8MOI. Dilute the SARS-CoV-2 (2019-nCoV) virus to a corresponding concentration with 2% cell maintenance solution, and then add it to a 24-well plate, so that the cell virus load in each well reaches the set infection dose. Chloroquine phosphate and hydroxychloroquine were diluted to corresponding concentrations with 2% cell maintenance solution, and added to the corresponding wells so that the final drug concentrations were 50 μM, 16.67 μM, 5.56 μM, 1.85 μM, 0.62 μM, 0.21 μM, 0.068 μM, respectively. μM, then placed in 37°C, 5% CO 2 The incubator continued to culture for 4...
Embodiment 3
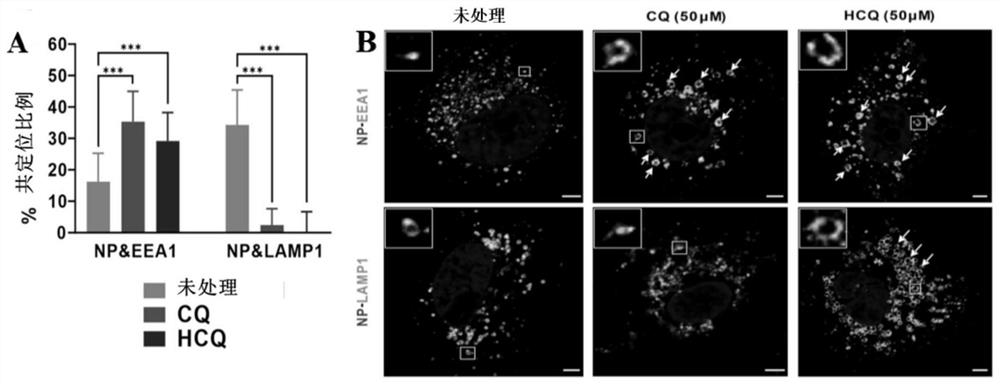
[0128] Embodiment 3: Chloroquine phosphate and hydroxychloroquine inhibit SARS-CoV-2 virus invasion mechanism experiment
[0129] (1) Experimental method
[0130] 1) Prepare chloroquine phosphate or hydroxychloroquine into 50 μM drug-containing culture solution with MEM culture solution containing 2% FBS, and then use the drug-containing culture solution to treat veroE6 cells for 1 h;
[0131] 2) Combine veroE6 cells with SARS-CoV-2 virus for 1 h at 4°C (MOI=10);
[0132] 3) Wash twice with PBS (purchased from Gibco, product number C10010500BT) to remove unbound virus particles, add fresh preheated MEM culture solution containing 2% FBS, and incubate at 37°C for 90min;
[0133] 4) Fix the cells, and use anti-viral NP protein antibody (red) (preserved by Wuhan Institute of Virology, Chinese Academy of Sciences) and anti-early endosomal protein EEA antibody (green) (purchased from Cell Signaling Technology Company, product number 48453) or Anti-lysosomal protein LAMP antibody ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com