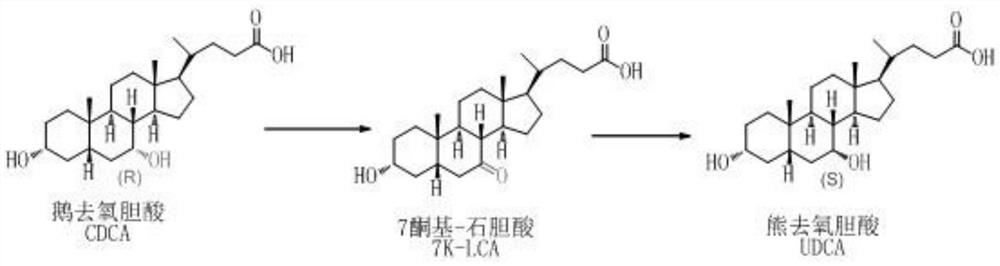
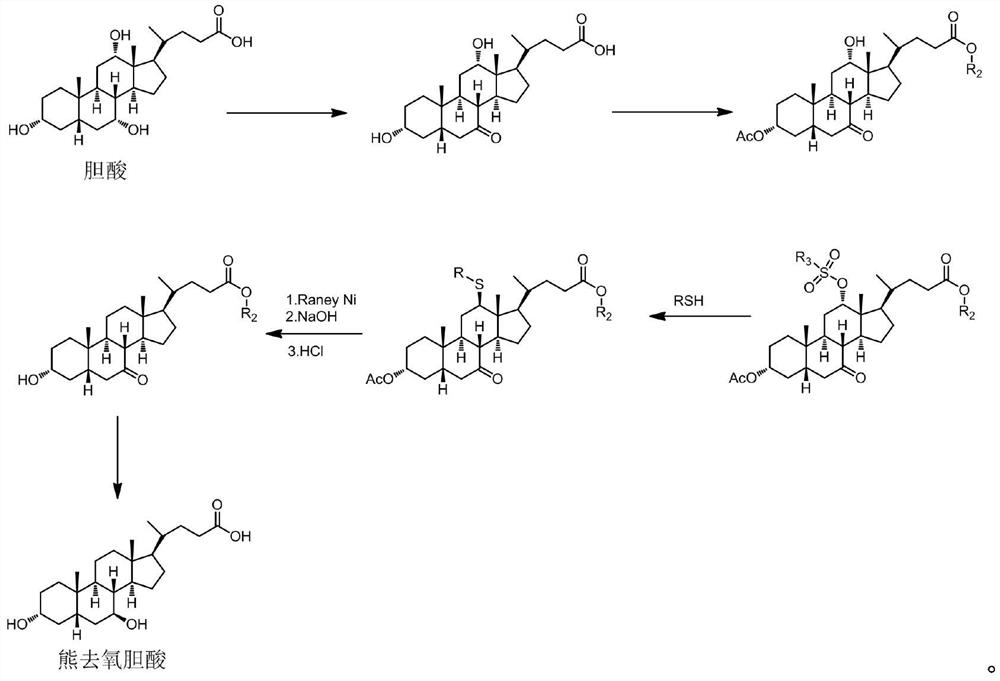
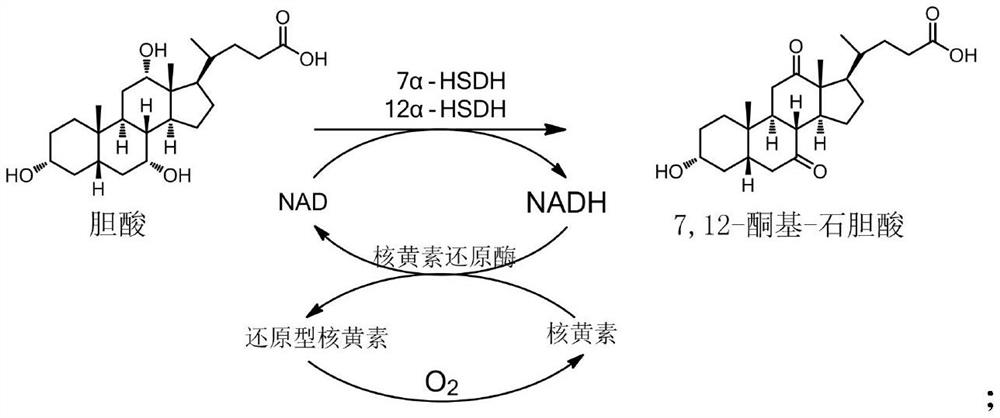
Preparation method of ursodesoxycholic acid
A technology of ursodeoxycholic acid and cholic acid, which is applied in the field of preparation of ursodeoxycholic acid, can solve the problems of high cost, pollution, and long process route, and achieve the effects of low cost, less side reactions, and shortened production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method of ursodeoxycholic acid, comprising the following preparation steps:
[0032] (1) Preparation of 7,12-keto-lithocholic acid
[0033] Add 25 grams of cholic acid to 175 ml of water, add 2.52 grams of sodium hydroxide, stir and dissolve to obtain an aqueous solution of cholic acid;
[0034] Prepare 1000ml of aqueous solution containing 7α-HSDH enzyme, 12α-HSDH enzyme, riboflavin reductase, and NAD coenzyme. The concentration of 7α-HSDH enzyme activity in the solution reaches 20U / ml, 12α-HSDH enzyme 20U / ml, Enzyme activity is 40U / ml, NAD coenzyme concentration is 0.04g / L; add 0.3g riboflavin, control temperature at 28±2°C, feed air at a flow rate of 1L / min; control pH at 8.0-8.3, add bile Aqueous acid solution, continue to ventilate and stir to react to generate 7,12-keto-lithocholic acid. After 2 hours of addition, the conversion rate is monitored by HPLC every hour until the conversion rate is ≥97%;
[0035] When the conversion rate is ≥97%, filter...
Embodiment 2
[0044] A preparation method of ursodeoxycholic acid, comprising the following preparation steps:
[0045] (1) Preparation of 7,12-keto-lithocholic acid
[0046] Add 50 grams of cholic acid to 350 ml of water, add 3.42 grams of sodium hydroxide, stir and dissolve to obtain an aqueous solution of cholic acid;
[0047] Prepare 1000ml of aqueous solution containing 7α-HSDH enzyme, 12α-HSDH enzyme, riboflavin reductase and NAD coenzyme. The concentration of 7α-HSDH enzyme activity in the solution reaches 40U / ml, 12α-HSDH enzyme 40U / ml, Enzyme activity 60U / ml, NAD coenzyme concentration 0.06g / L; then add 0.5g riboflavin, control the temperature at 28±2°C, let in air, control the dissolved oxygen at 10%~70%; control the pH at 8.0~ 8.3, add the aqueous solution of cholic acid, continue to ventilate and stir the reaction to generate 7,12-keto-lithocholic acid, after 2 hours after the addition, monitor the conversion rate by HPLC every hour until the conversion rate is ≥98%;
[0048] Wh...
Embodiment 3
[0057] A preparation method of ursodeoxycholic acid, comprising the following preparation steps:
[0058] (1) Preparation of 7,12-keto-lithocholic acid
[0059] Add 75 grams of cholic acid to 500 ml of water, add 7.15 grams of sodium hydroxide, stir and dissolve to obtain an aqueous solution of cholic acid;
[0060] Prepare 1000ml of aqueous solution containing 7α-HSDH enzyme, 12α-HSDH enzyme, riboflavin reductase, and NAD coenzyme. The concentration of 7α-HSDH enzyme activity in the solution reaches 80U / ml, 12α-HSDH enzyme 80U / ml, Enzyme activity 100U / ml, NAD coenzyme concentration 0.08g / L; then add 0.6g riboflavin, control the temperature at 28±2°C, feed air at a flow rate of 1.5L / min; control the pH at 8.0-8.3, add Cholic acid aqueous solution, continue to ventilate and stir to react to generate 7,12-keto-lithocholic acid. After 2 hours of addition, the conversion rate is monitored by HPLC every hour until the conversion rate is ≥98%;
[0061] When the conversion rate is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com