CD73 enzyme activity-related antigen epitope and preparation method of specific antibody targeting to epitope
An antigen epitope and specific technology, applied in the field of medicine, can solve the problems of reducing the possibility of becoming a drug, lack of blocking its enzyme activity, etc., to shorten the time of antigen preparation, wide application prospects and practical value, and reduce the concentration of adenosine Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Identification of antigenic epitopes related to CD73 enzymatic activity
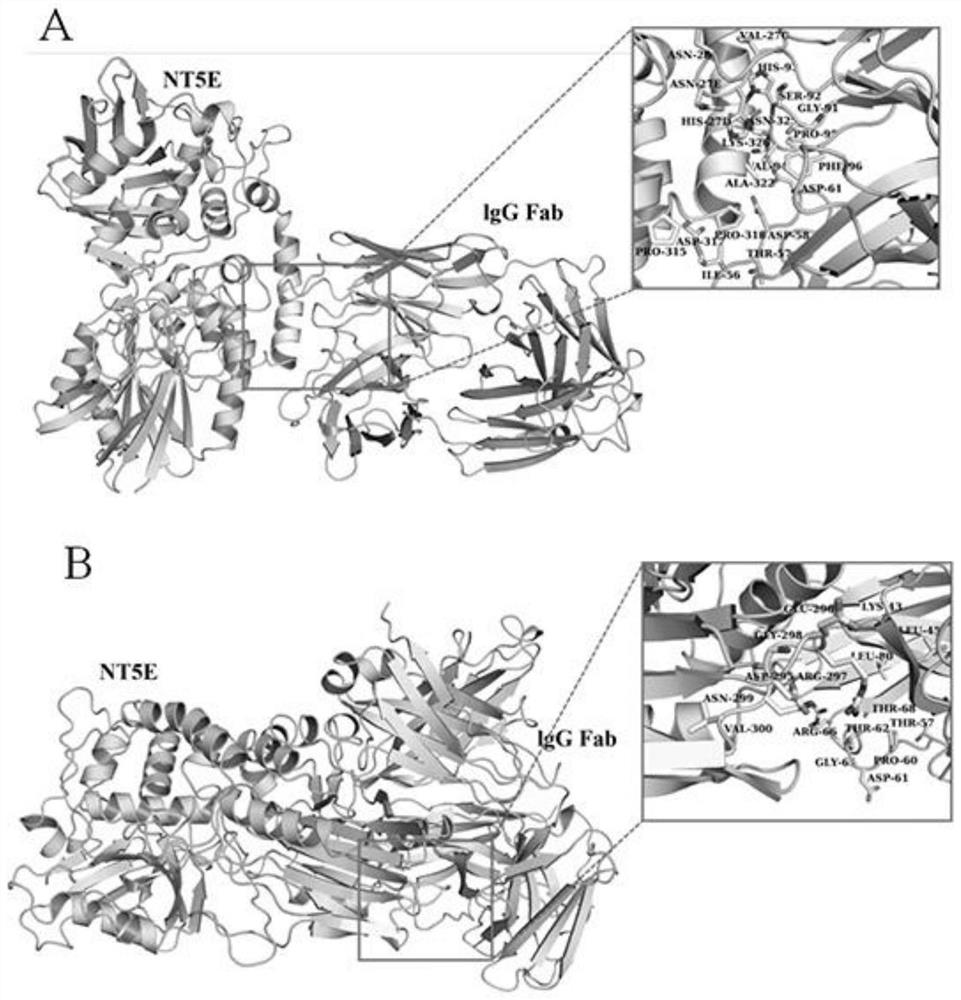
[0052] We simulated the spatial conformation of the CD73 enzymatic activity-related epitope binding to the antibody in a 3D environment through the biological modeling Docking Model method. (1) Obtain the crystal structure of NT5E (CD73) from the Program Database, and the protein IDs are 4H2F and 1CLY, respectively. (2) With the help of Schrodingger 2015 software, the following processes were performed on the two protein structures: adding the side chain of amino acid residues; adding the missing loop part in the crystal structure; assigning the protonation state of amino acid residues and the situation of applying the OPLS2005 force field Next, optimize the entire protein structure. (3) Use ZDOCK online calculation (http: / / zdock.umassmed.edu / ) for the docking of IgG Fab and NT5E. During the docking process, NT5E is selected as the receptor, and 1CLY is set as the ligand. The result i...
Embodiment 2
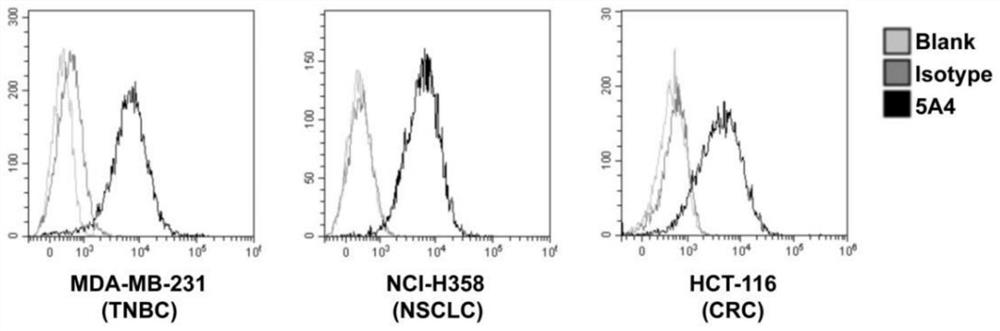
[0053] Example 2: Preparation of monoclonal antibodies against CD73 enzymatic activity-related epitopes
[0054] 1 Synthesis of peptide-carrier protein conjugates
[0055] Two polypeptides shown as SEQ ID NO: 1 and SEQ ID NO: 2 were respectively synthesized by peptide synthesis technology, and the HPLC detection purity was above 95%. Using the bifunctional coupling agent EDC, the -COOH on the above-mentioned synthetic peptide was directly combined with the -NH on the bovine serum albumin (BSA) and hemocyanin (KLH) respectively. 2 Coupling was carried out at 100:1 (molar ratio), and the coupling efficiency was identified by HPLC after removing the free polypeptide, that is, a polypeptide-BSA and polypeptide-KLH conjugate was obtained.
[0056] 2 Polypeptide-BSA conjugate immunized mice
[0057] Five Balb / c mice weighing 18-22 g and 4 weeks old were selected. Use the polypeptide-BSA conjugate as the antigen, the first immunization concentration is 1mg / mL, the mouse immunizati...
Embodiment 3
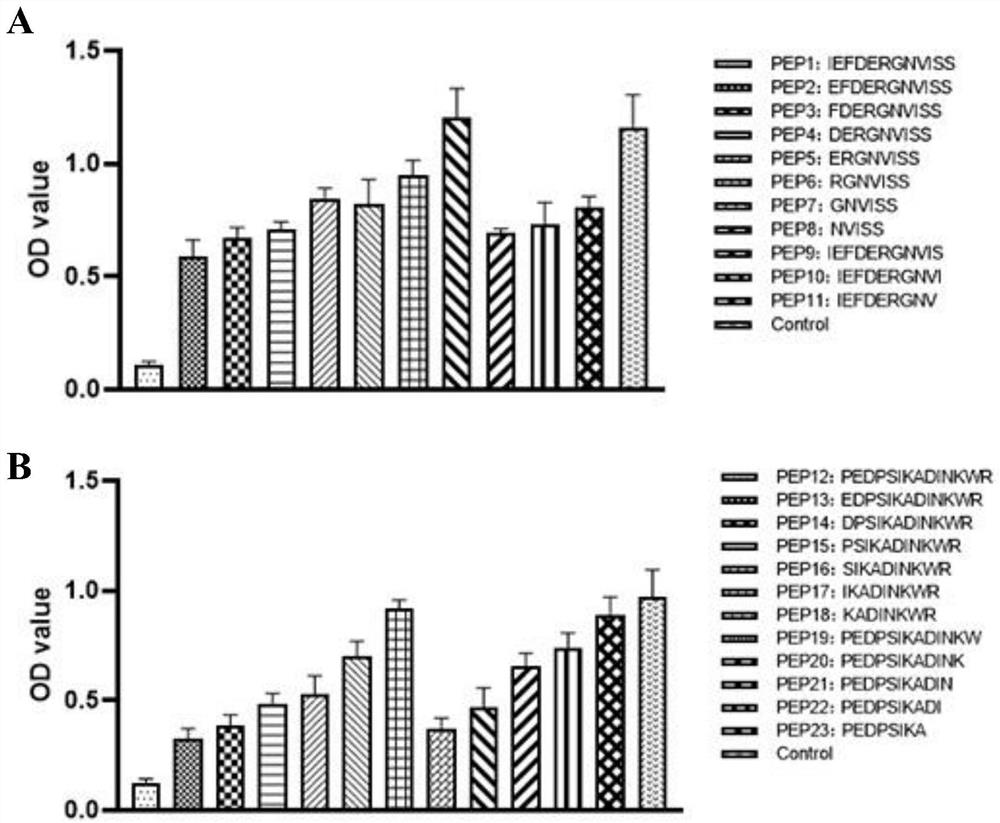
[0067] Example 3: Anti-CD73 enzymatic activity-related epitope-specific monoclonal antibody recognition amino acid sequence identification
[0068] (1) Coating plate: Dilute the two polypeptide-KLH conjugates of SEQ ID NO:1 or SEQ ID NO:2 to 2.5 μg / mL with coating buffer, add 100 μL / well, overnight at 4°C, the next day, discard the solution in the well, wash 3 times with 1×TBST washing buffer, and pat dry;
[0069] (2) Blocking: add 100 μL of 1% BSA to each well for blocking, incubate at 37°C for 1 hour, discard the blocking solution;
[0070] (3) Adding samples: add 50 μL / well of the above-mentioned 5A4 or 8-5 hybridoma culture supernatant+50 μL / well of 1% BSA or 50 μL / well of the above-mentioned 5A4 or 8-5 hybridoma culture supernatant+50 μL / well 1 mg / mL naked peptide in each well (Table 2 is the amino acid sequence of different naked peptides), each set up 3 duplicate wells, incubate at 37°C for 120 min, pat dry, wash 3 times with 1×TBST washing buffer, and pat dry;
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com