Preparation method of Au@Ag@AgCl nanoparticles and application thereof in colorimetric detection of ammonia
A nanoparticle and nanoparticle technology, applied in the field of Au@Ag@AgCl nanoparticle probe for ammonia colorimetric detection, can solve the problems of poor probe sensitivity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
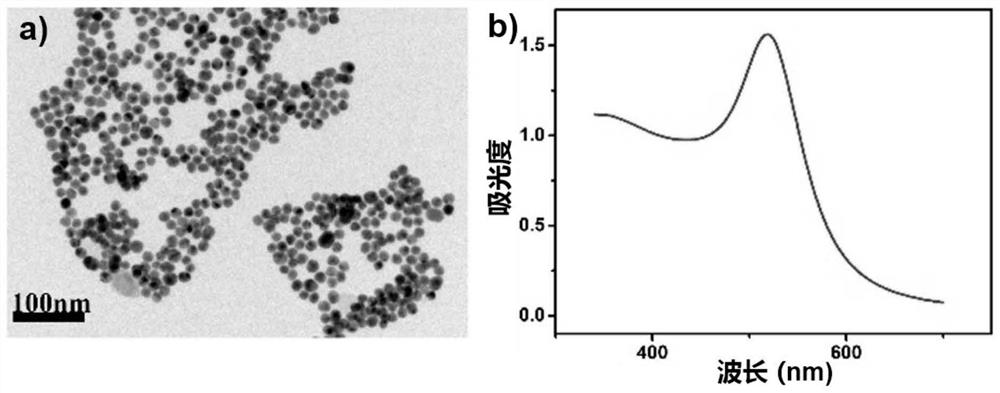
[0043] Example 1: The detection effect of the Au@Ag@AgCl nanoparticle probe example prepared by the present invention on ammonia solutions with series concentrations is given below. A series of ammonia solutions (0-5000 μM) were prepared, and the Au@Ag@AgCl nanoparticle probe solution was added. After reacting for 10 minutes at room temperature (25°C±2°C), photographs were taken and UV-visible absorption spectra were scanned. Figure 11 (a) shows that when the ammonia water is in the range of 0-1600 μM, the absorbance value of the characteristic absorption peak at 510 nm of the Au@Ag@AgCl nanoparticle probe solution gradually increases with the increase of the ammonia water concentration, and the change value of the absorbance It has a good linear relationship with the ammonia concentration ( Figure 11 (b)), the linear correlation coefficient reaches 0.997, and the minimum detection concentration calculated according to the linear equation is 6.4 μM; Figure 12 (a) shows tha...
Embodiment 2
[0044] Embodiment 2: The following is a comparison of the response effects of the Au@Ag@AgCl nanoparticle probe embodiment of the present invention to ammonia gas and other 10 interfering reagents / gases. Figure 13 It shows that the response signal of the Au@Ag@AgCl nanoparticle probe of the present invention to ammonia is 8.12-139 times that of all other 10 interfering reagents / gases, indicating that the method has high specificity to ammonia.
Embodiment 3
[0045] Embodiment 3: The following is an embodiment of the Au@Ag@AgCl nanoparticle probe of the present invention for detecting an actual gas sample. In order to test the feasibility of this method in the detection of ammonia content in actual samples, it was applied to the detection of ammonia content in simulated polluted gases. Aqueous ammonia solution (9 mM, 10 mL) was injected into a constant volume (20 L) gas sampling bag, which was then filled with air by an air pump to prepare contaminated gas. It is then divided into 4 gas sampling bags (5 liters) for use. Four more gas sampling bags (5 liters) were used to collect fresh air. Various amounts of aqueous ammonia aqueous standard solutions (0.17, 0.34, 0.51, 0.68, 1.02mg / L) were injected into the gas sampling bags. Before analysis, the air sampling bag was heated to 50°C to ensure uniform diffusion of ammonia gas. Gas samples with different concentrations were drawn into 20 mL medical syringes into which 5 mL of Au@Ag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com