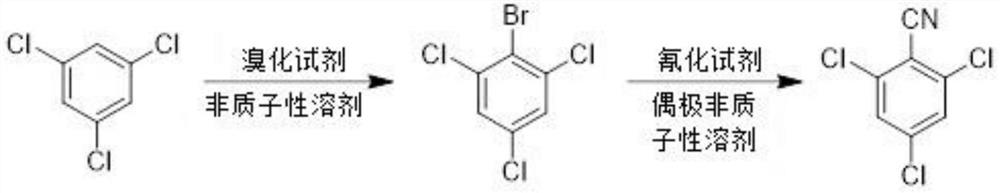
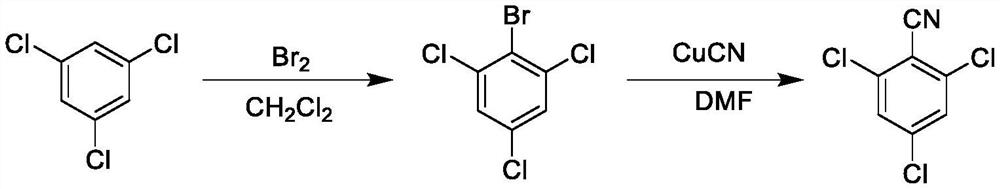
Preparation method of 2, 4, 6-trichlorobenzonitrile
A technology of trichlorobenzonitrile and trichlorobenzene, which is applied in the field of preparation of 2,4,6-trichlorobenzonitrile, can solve the problems of high cost, environmental pollution, and large amount of three wastes, and achieve low cost and simple and easy reagents The effect of gaining and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Synthesis of 2-bromo-1,3,5-trichlorobenzene
[0032] Dissolve s-trichlorobenzene (100 g, 1.00 eq.) in dry dichloromethane (1200 ml), add ferric chloride, raise the temperature to 20-30°C, add liquid bromine (96.8 g, 1.1 eq.), dropwise After the addition, continue to maintain 20-30 ° C, react for 5 hours (HPLC detection reaction raw materials ≤ 2.0%), cool down to room temperature, pour the reaction system into 15% sodium bisulfite solution (600 g) to quench the reaction , after standing for stratification, separate the organic phase, then wash the organic phase with 15% aqueous sodium bisulfite solution (300 g) and saturated brine (500 g) successively, then stand for stratification, and separate the organic phase again , the separated organic phase was dried and concentrated to obtain 2-bromo-1,3,5-trichlorobenzene product (light yellow solid, about 140.4 g), with a yield of 97.79% and a purity of 94.50%.
[0033] (2) Synthesis of 2,4,6-trichlorobenzonitrile
[00...
Embodiment 2
[0038] (1) Synthesis of 2-bromo-1,3,5-trichlorobenzene
[0039] Dissolve s-trichlorobenzene (150 g, 1.0 eq) in dry dichloromethane (1200 ml), add ferric chloride, raise the temperature to 20-30°C, add liquid bromine (158.2 g, 1.2 eq), dropwise After the addition, continue to maintain 20-30 ° C, react for 5 hours (HPLC detection reaction raw materials ≤ 2.0%), cool down to room temperature, pour the reaction system into 15% sodium bisulfite solution (900 g) to quench the reaction , after standing for stratification, separate the organic phase, then wash the organic phase with 15% aqueous sodium bisulfite solution (450 g) and saturated brine (750 g) successively, then stand for stratification, and separate the organic phase again , the separated organic phase was dried and concentrated to obtain 2-bromo-1,3,5-trichlorobenzene product (pale yellow solid, about 211.4 g), with a yield of 98.20% and a purity of 96.20%.
[0040] (2) Synthesis of 2,4,6-trichlorobenzonitrile
[0041]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com