Chitosan-coated solid lipid nanoparticles for promoting absorption of pentacyclic triterpenoids and preparation method thereof
A solid lipid nanometer, pentacyclic triterpenoid technology, applied in the directions of pharmaceutical formulations, drug combinations, drug delivery, etc., can solve the problems of difficult absorption of pentacyclic triterpenoids, difficulty in obtaining apolipoproteins, etc., and achieve sustained release. And the effect of drug absorption, promoting drug absorption, and promoting absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Construction and characterization of embodiment 1 drug delivery carrier
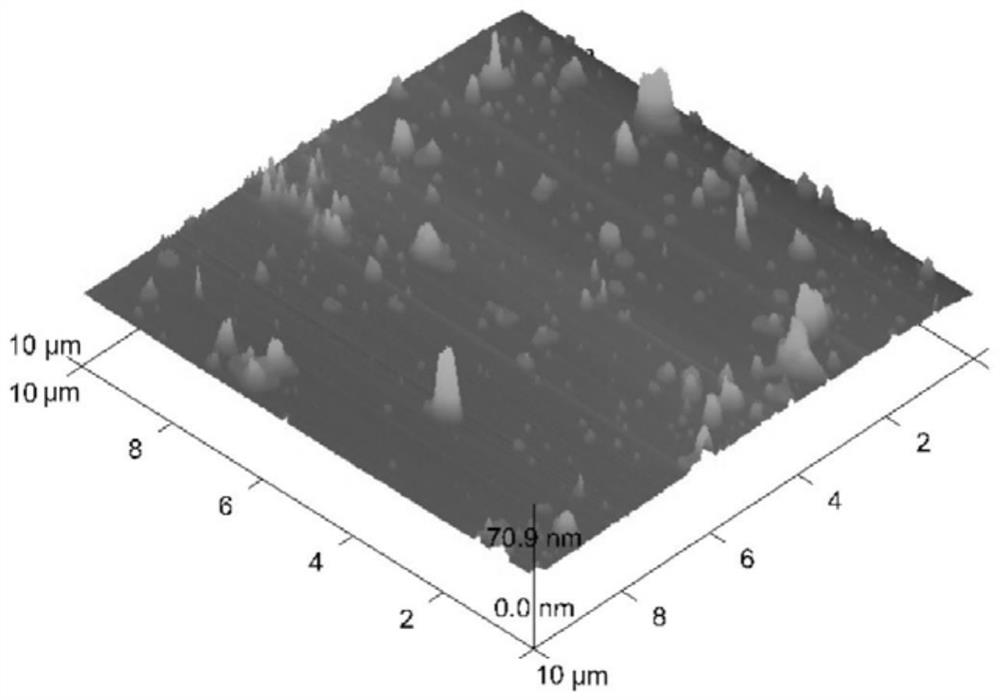
[0045] Precisely weigh the prescribed amount of oleanolic acid or ursolic acid and dissolve it in an appropriate amount of ethanol, add the prescribed amount of glyceryl monostearate and stearic acid, and melt completely at 75-80°C to form an oil phase solution. Accurately weigh an appropriate amount of hydroxyethyl chitosan and dissolve it into a solution; then add an appropriate amount of poloxamer until it is completely dissolved to form an aqueous solution. Heat the water phase to 75-80°C, and drop the oil phase under magnetic stirring. Continue to stir at 50-80°C for 5min-120min, then quickly place it in an ice bath for ultrasonication, and pass through a 0.45μm microporous membrane to obtain drug-loaded biomimetic nanoparticles.
[0046] Drop the drug-loaded biomimetic nanoparticles on the mica sheet, wait for the water to dry up, and observe under the atomic force microscope, as shown in ...
Embodiment 2
[0048] Embodiment 2 drug loading process optimization
[0049] Taking oleanolic acid as a model drug and hydroxyethyl chitosan as a chitosan material, the probe ultrasonic method (500W, ultrasonic 15s, stop 15s, 10min in total), high-speed stirring method (10000rpm, 5min), ultrasonic method (300W, 30min) and other preparation methods on the particle size, potential, and encapsulation efficiency of nanoparticles.
[0050] Table 1 Drug-loaded micelles obtained by different preparation methods (n=3)
[0051] Preparation Encapsulation % Particle size (nm) Potential (mV) Probe Ultrasound 84.3±3.2 217.2±5.4 16.3±2.5 High-speed stirring method 88.5±2.8 224.6±8.3 15.2±2.8 Ultrasound 93.2±2.6 257.2±7.2 16.3±2.1
[0052] The results show that the nanoparticles obtained by the ultrasonic method have the highest encapsulation efficiency, slightly larger particle size, and more stable potential, so the ultrasonic method is used to prepare them a...
Embodiment 3
[0060] Embodiment 3 in vitro release behavior
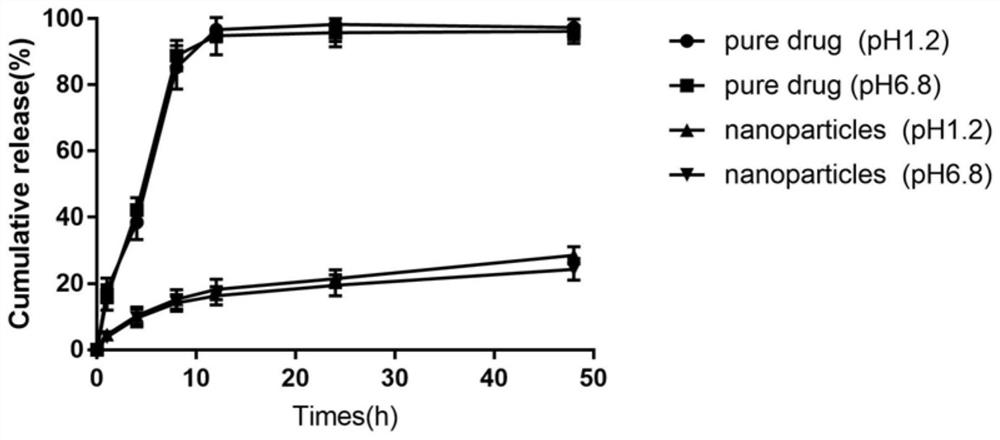
[0061] Take the drug-loaded biomimetic nanoparticles and the original drug, respectively, 5 mg in terms of oleanolic acid, put them into a pre-treated dialysis bag (the molecular mass cut-off is 7000-14000), and place the dialysis bag in 200 mL to release In the medium (buffer solutions of pH 1.2 and pH 6.8, both containing 0.5% SLS), shake (100r / min) in a constant temperature water bath at (37±0.5)°C, and wait for 1, 4, 8, 12, 24 and 48 hours respectively Take a sample of 1mL, and add an equal amount of release medium at the same temperature. The sample is filtered with a 0.45 μm microporous membrane, and the drug content is determined by HPLC to be calculated. The relationship between the cumulative release percentage and time is shown in image 3 . The results show that the drug release of nanoparticles in the dialysis bag is obviously slowed down, and it has a good sustained release effect under different pH conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com