Hydrophobic deep-eutectic solvent used for separating nickel and cobalt ions, preparation method of hydrophobic deep-eutectic solvent and method for separating nickel and cobalt ions
A deep eutectic solvent and hydrophobic technology, applied in the field of non-ferrous metal metallurgy, can solve problems such as potential safety hazards, volatile diluents, and pH value effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
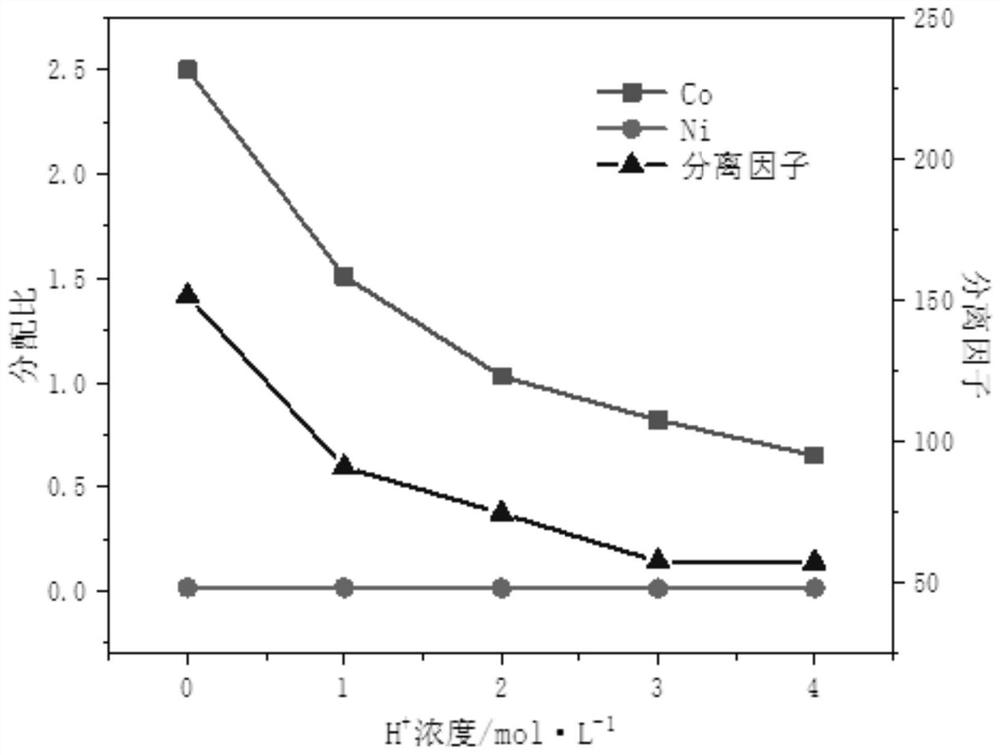
Image
Examples
Embodiment 1
[0039] This embodiment takes trioctylmethylammonium chloride and menthol to prepare a hydrophobic deep eutectic solvent to extract and separate nickel and cobalt ions as an example. The specific steps are as follows,
[0040] 1) Mix the hydrogen bond acceptor and the hydrogen bond donor at a molar ratio of 1:1, place in a heating device at a constant temperature to 70°C, heat and stir for 1 hour, until the mixture becomes a uniform transparent liquid, and obtain two components with hydrogen Hydrophobic eutectic mixture combined in bond form as cobalt extractant.
[0041]2) Add sodium chloride to the nickel sulfate solution of 0.2mol / L and the cobalt sulfate solution of 0.2mol / L respectively until the chloride ion concentration is 4mol / L; Add a hydrophobic deep eutectic solvent to the cobalt sulfate solution, mix the aqueous phase and the organic phase with a vortex mixer, and the mixing time is 1min. After the extraction process reaches equilibrium, the phases are separated by...
Embodiment 2
[0046] In this embodiment, the preparation of trioctylmethylammonium bromide and menthol to extract and separate nickel and cobalt ions with a hydrophobic deep eutectic solvent is an example. The specific steps are as follows:
[0047] 1) Mix the hydrogen bond acceptor and the hydrogen bond donor according to the molar ratio of 1:1.5, place in a heating device at a constant temperature to 60°C, and heat and stir for 1.5 hours until the mixture becomes a uniform transparent liquid to obtain two components: Hydrophobic eutectic mixture in the form of hydrogen bonding as cobalt extractant.
[0048] 2) Add sodium chloride to the nickel sulfate solution of 0.2mol / L and the cobalt sulfate solution of 0.2mol / L respectively until the chloride ion concentration is 5mol / L; A hydrophobic deep eutectic solvent was added to the cobalt sulfate solution, and the aqueous phase and the organic phase were mixed with a vortex mixer for 1.5 minutes. After the extraction process reached equilibriu...
Embodiment 3
[0052] In this embodiment, taking trioctylmethylammonium bromide and thymol to prepare a hydrophobic deep eutectic solvent to extract and separate nickel and cobalt ions as an example, the specific steps are as follows,
[0053] 1) Mix the hydrogen bond acceptor and the hydrogen bond donor according to the molar ratio of 1:1.25, place in a heating device at a constant temperature to 80°C, heat and stir for 1 hour, until the mixture becomes a uniform transparent liquid, and obtain two components with hydrogen Hydrophobic eutectic mixture combined in bond form as cobalt extractant.
[0054] 2) Add sodium chloride to the 0.2mol / L nickel sulfate solution and 0.2mol / L cobalt sulfate solution respectively until the chloride ion concentration is 4mol / L; add hydrophobic eutectic at a ratio of 1:1 For the solvent, use a vortex mixer to mix the aqueous phase and the organic phase. The mixing time is 2 minutes. After the extraction process reaches equilibrium, the phases are separated by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com