A method for constructing an animal model of intestinal mucositis
A technology of animal models and construction methods, applied in the field of biomedicine, which can solve problems such as lack of typicality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A method for constructing an animal model of intestinal mucositis
[0036] 1. Experimental reagents: Harris hematoxylin solution (contains 1g of hematoxylin in solution A and 10ml of anhydrous alcohol, and 20g of potassium aluminum sulfate in solution B and 200ml of ultrapure water)
[0037] Preparation method: weigh KAl (SO 4 ) 2 ·12H 2 O 36.72g and hematoxylin 1g were dissolved and mixed respectively, heated and boiled until the solid dissolved, slowly added 0.5g of mercuric oxide, the mixed liquid was rapidly cooled and cooled, and the liquid was filtered using a 0.45mm filter membrane after cooling. When ready to use, mix and configure according to the ratio of adding 6 ml of glacial acetic acid and 10 ml of glycerol (glycerol) per 100 ml.
[0038] Bouin's fixative: Mix 75ml of picric acid, 25ml of 40% formaldehyde solution and 5ml of glacial acetic acid.
[0039] 2. Selection and grouping of experimental animals: 20 SPF grade BALB / c mice aged 9-10 weeks were ra...
Embodiment 2
[0070] Simultaneous administration of a large area of the flora in the intestinal tract can lead to physical discomfort or death of the mouse, while the administration of a small dose may cause the administered flora to be killed by the body's own immune system, resulting in the failure of the flora administration. Based on the long-acting and uninterrupted microflora application mode, the present invention adopts a bacterial liquid capsule. The bacterial liquid capsule is sealed and encapsulated by a polymer permeable membrane, which can slowly release the encapsulated bacterial liquid through the osmosis or self-decomposition of the permeable membrane. Since the size of bacteria is generally between 0.5 and 7 μm, the pore size of the controlled-release membrane or sustained-release membrane should be set according to the bacterial liquid to be wrapped. The permeable membrane pore size can be 3 μm. When the encapsulated bacterial solution is a mixed bacterial solution, the...
Embodiment 3
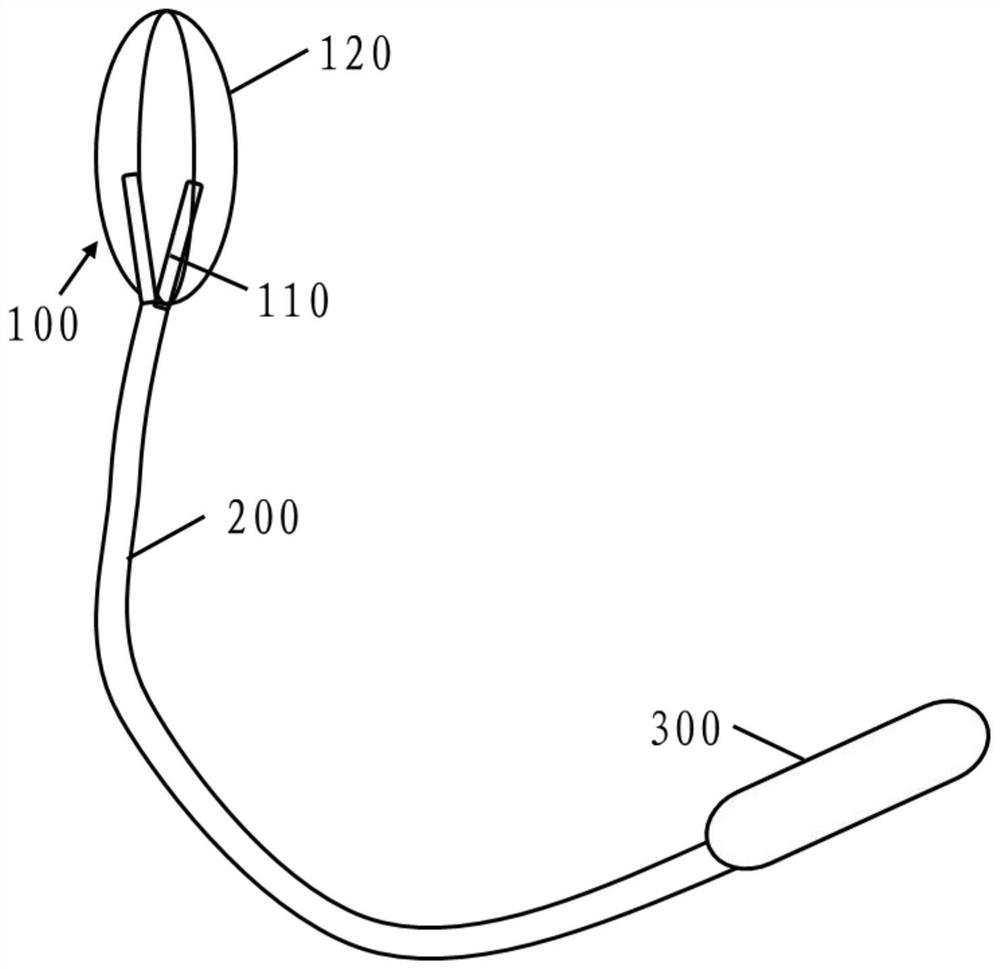
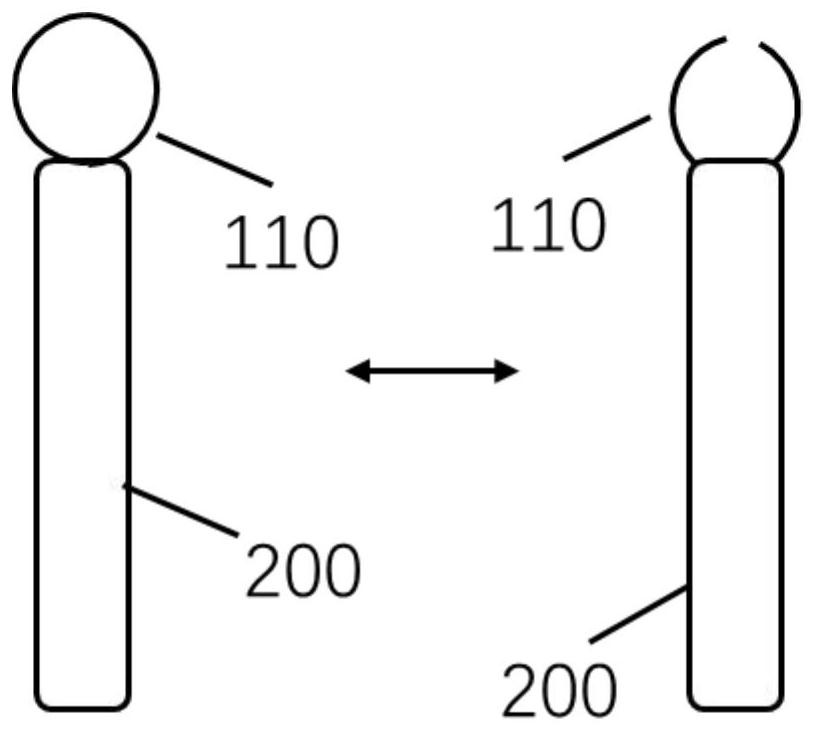
[0075] This embodiment discloses a capsule administration device. The applicator includes a fixing unit 100 , a connecting rod 200 and a handle 300 . The fixing unit 100 can hold the capsule, so that the fixing unit 100 can release the capsule when the capsule is in a predetermined position in the intestine, such as figure 1 The connecting rod 200 can change with the bending shape of the intestinal tract when entering the intestinal tract, and the connecting rod 200 is provided with electronic detection or scale, so that the user can know the length of the applicator entering the mouse and release it at a fixed position Capsules, since the user cannot clearly perceive the location of the capsule entering the intestine when administering the capsule to the mouse, it is necessary to design a tool that can assist the user to understand the depth of the intestine where the capsule is located. The handle 300 helps control the applicator by the user's hand. The handle 300 is provi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com