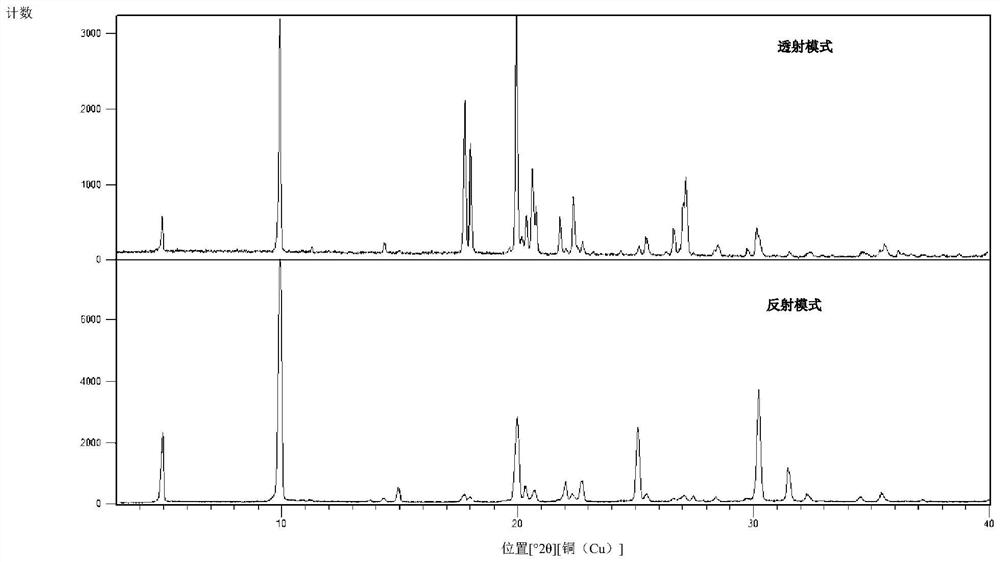
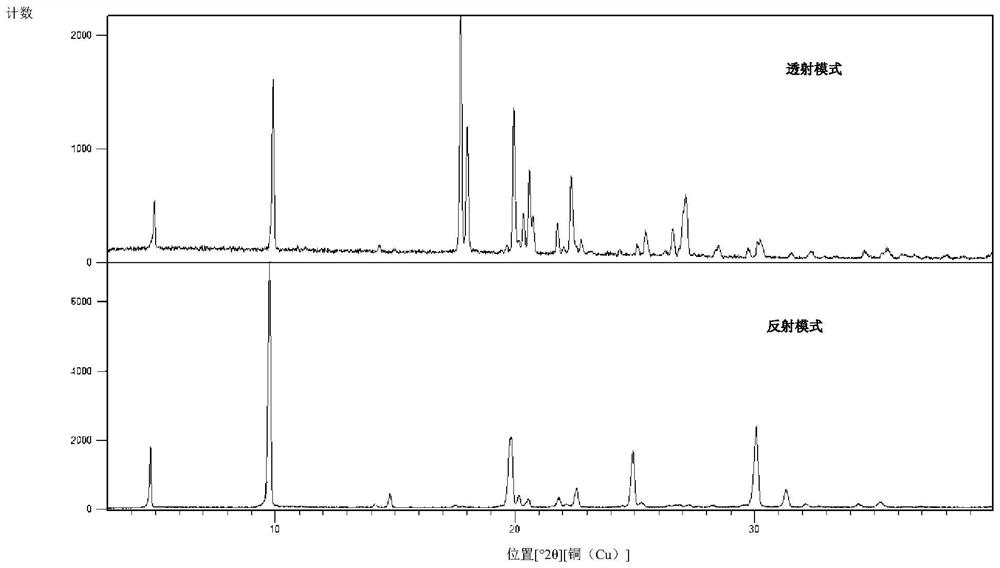
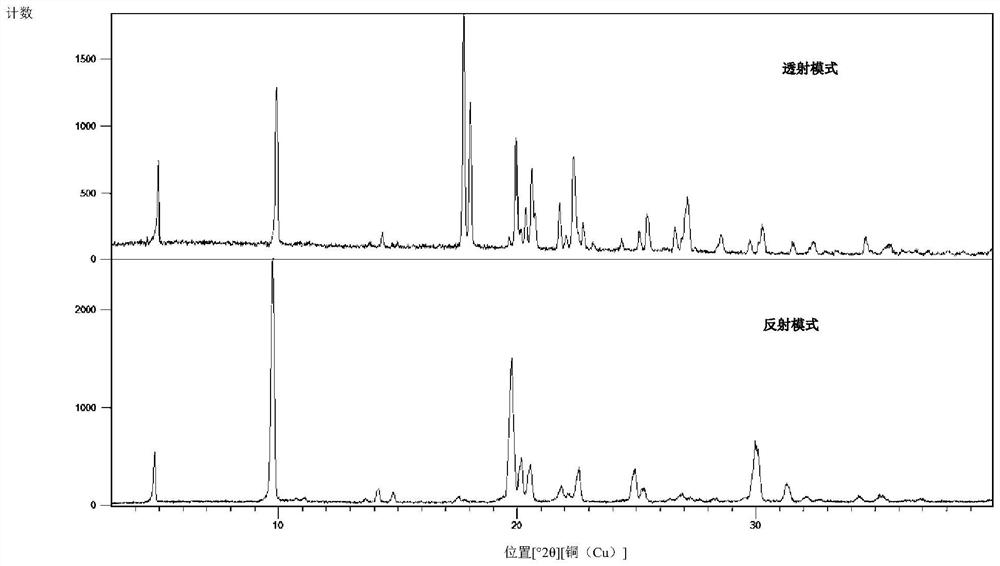
Herbicide and production method for intermediate thereof
A manufacturing method and a halogenating agent technology, applied in the field of sulfone derivatives, can solve problems such as chemical damage to crops, separation difficulties, and quality decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0890]
[0891] [Chemical 44]
[0892] WO2004 / 013106A1, Example 14
[0893]
[0894] However, the compound of formula (2) is preferably produced by the method of the present invention. That is, when X in formula (2) 1 In the case of a halogen atom, the compound of the formula (2) is preferably produced by a method including the step i described in this specification.
[0895] In formula (2), R 1 , R 2 and R 3 as defined above. In formula (2), R 1 , R 2 and R 3 Examples, preferred examples, more preferred examples and particularly preferred examples are as described above.
[0896] X in formula (2) 1 for the leaving group. As long as it functions as a leaving group in the reaction of step ii, X in formula (2) 1 Can be any atom or group of atoms.
[0897] From the viewpoints of productivity, availability, price, etc., X in formula (2) 1 Preferred examples include halogen atoms, (C1-C4) alkylsulfonyloxy, (C1-C4) haloalkylsulfonyloxy, benzenesulfonyloxy optiona...
Embodiment 1
[1321] 3-[(5-Difluoromethoxy-1-methyl-3-trifluoromethylpyrazol-4-yl)methylthio]-4,5-dihydro-5,5-dimethyliso Production of oxazole (compound 4-a)
Embodiment 1-1
[1323] (Process i)
[1324] Production of 4-chloromethyl-5-difluoromethoxy-1-methyl-3-trifluoromethylpyrazole (compound 2-a)
[1325] [Chemical 53]
[1326]
[1327] To 5-difluoromethoxy-4-hydroxymethyl-1-methyl-3-trifluoromethylpyrazole (1-a) (46.7 g, purity: 68.6%, containing acetonitrile, 0.13 mol, 100 mol %), thionyl chloride (17.0 g, 0.14 mol, 110 mol %) was added dropwise over 1 hour at an internal temperature of 20°C to 30°C. After the dropwise addition, the mixture was aged at an internal temperature of 20°C to 30°C for 1 hour. After the reaction was completed, nitrogen was purged into the reaction mixture for 30 minutes to remove excess thionyl chloride, and ethyl acetate (78 mL, 0.6 L / mol) was added. The ethyl acetate solution of the obtained title compound (2-a) was 134 g.
[1328] (Process ii)
[1329] 3-[(5-Difluoromethoxy-1-methyl-3-trifluoromethylpyrazol-4-yl)methylthio]-4,5-dihydro-5,5-dimethyliso Production of oxazole (compound 4-a)
[1330] [Chemica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com