A kind of terpyridine ligand of nitrogen mustard and its preparation method and application
The technology of terpyridine and nitrogen mustard is applied in the field of pharmaceutical intermediates and anti-tumor drugs, which can solve problems such as large molecular structure, and achieve the effects of easy availability of raw materials, few operation steps and good anti-tumor activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
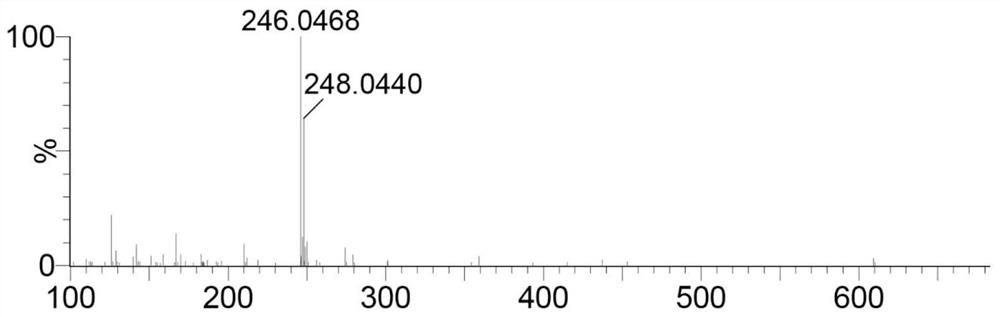
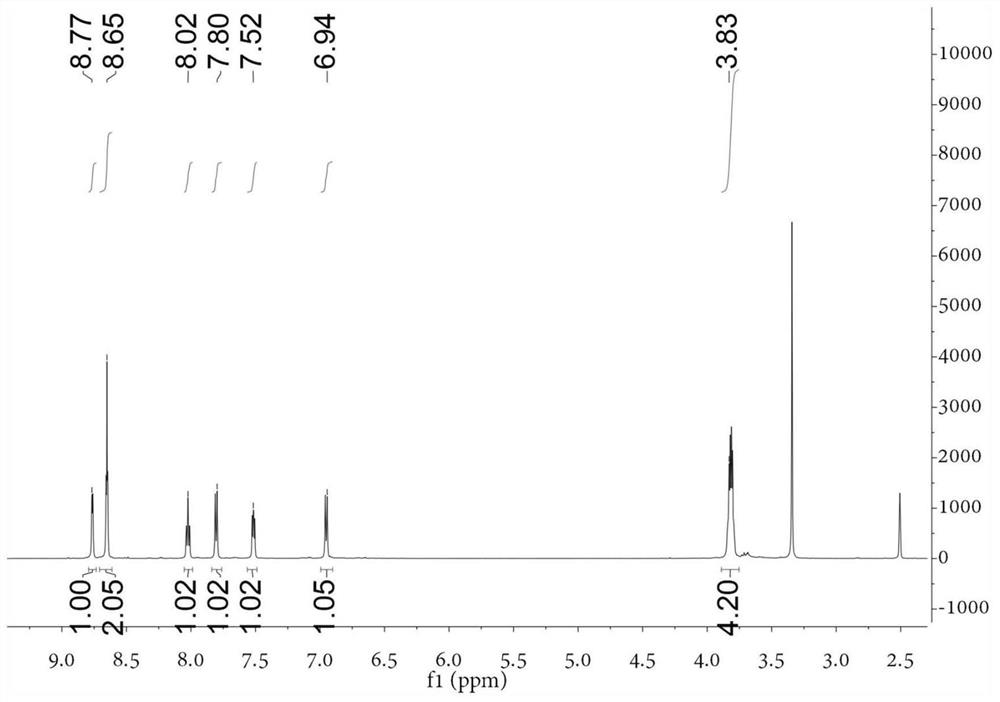
[0055] Take the following synthetic route to prepare 4-(4-[bis(β-chloroethyl)amino]phenyl)-2,2':6',2-terpyridine (compound L1):
[0056]
[0057] step 1.
[0058] Synthesis of 4-[bis(β-chloroethyl)amino]benzaldehyde:
[0059]
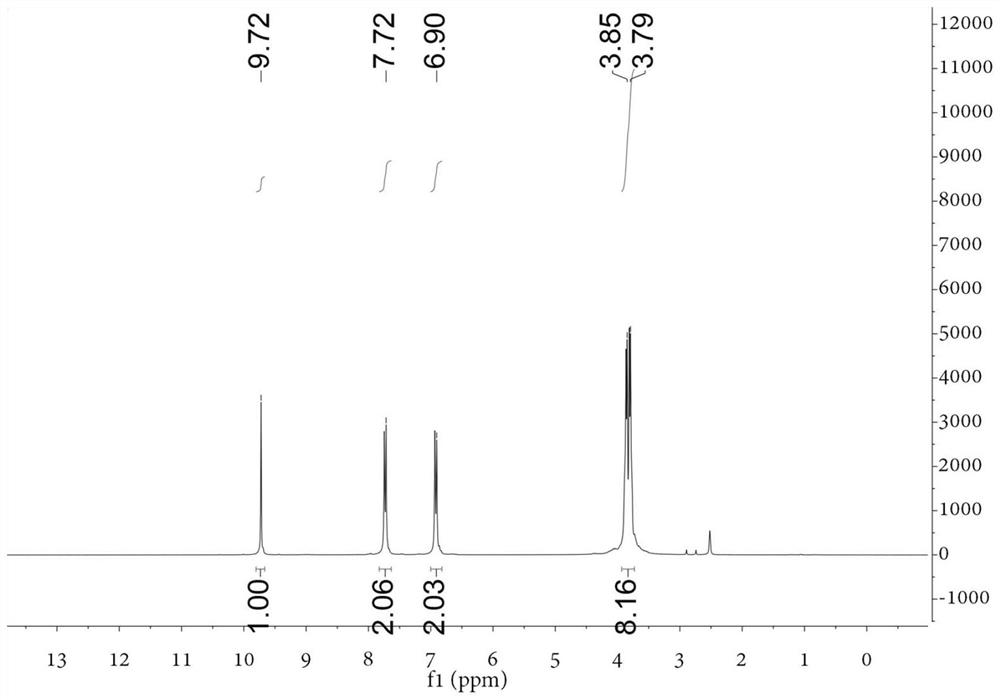
[0060] Add dry DMF 102.2mmol (7.46g) to the round bottom flask, and slowly add POCl dropwise while stirring in an ice-water bath 3 45.5mmol (6.97g), after the dropwise addition, continue to react in the ice bath for 30min. Then add N,N-bis(2-hydroxyethyl)-aniline 13.8mmol (2.50g) in DMF 13.8mmol (1.01g) solution, and react at 100°C for 3h. After cooling to room temperature, it was poured into 200 mL of ice water, and 1 mol / L NaOH was added dropwise with stirring to adjust to neutrality. After suction filtration, the filter cake was washed twice with a small amount of cold ethanol / water mixture (v:v=1:1), and finally recrystallized with ethanol / dichloromethane (v:v=1:1) mixture to obtain shallow Yellow solid, yield 85%. figure 1 For the H NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com