A kind of chondroitin sulfate modified natamycin eye drops and preparation method thereof
A technology of chondroitin sulfate and natamycin, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., which can solve the problem of the large number of raw materials and the long-term efficacy of eye drops preparation technology Short, short ocular surface half-life and other issues, to achieve the effect of simple preparation process, enhanced compliance, and quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
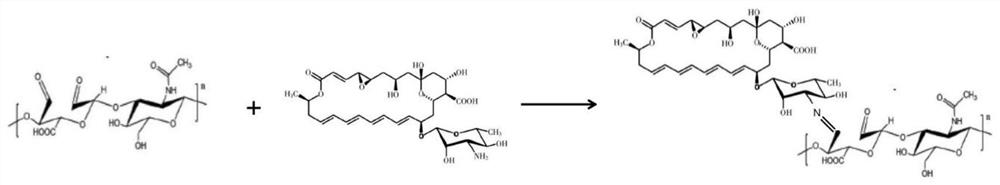
[0039] The components and contents of the chondroitin oxysulfate-modified natamycin eye drops involved in this example are: in every 100 ml of phosphate buffer solution, the chondroitin oxysulfate-natamycin freeze-dried powder added The content is 10-80mg. The preparation method steps of the chondroitin oxysulfate-modified natamycin eye drops include three process steps: preparing chondroitin oxysulfate, preparing chondroitin oxysulfate-natamycin pharmaceutical granules, and preparing eye drops:
[0040] The first step is to prepare oxychondroitin sulfate:
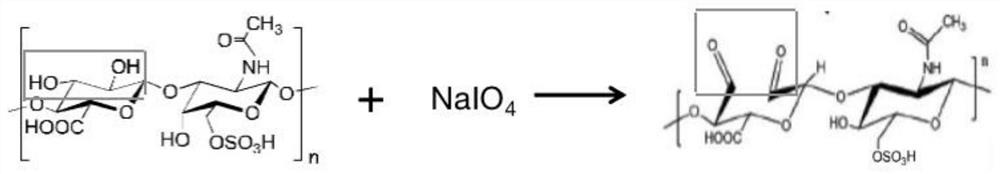
[0041] (2) put 2~10g of chondroitin sulfate in every 100ml of deionized water, after dissolving, add 1~5g of sodium periodate, and continue stirring reaction at room temperature and dark condition for 12 hours to generate chondroitin sulfate oxide, The reaction formula is:
[0042]
[0043] (3) 0.5~2ml of ethylene glycol was added to the reaction solution obtained in step (1), and stirred for 5 minutes to quench the s...
Embodiment 2
[0053] The components and contents of the chondroitin oxysulfate-modified natamycin eye drops involved in this example are: in every 100 ml of phosphate buffer solution, the chondroitin oxysulfate-natamycin freeze-dried powder added The content is 40mg. The eye drops are prepared according to the process steps of Example 1:
[0054] The first step is to prepare oxychondroitin sulfate:
[0055] (1) 5g of chondroitin sulfate was dissolved in 100ml of deionized water, then 3.5g of sodium periodate was added, and the reaction was continuously stirred for 12 hours at room temperature and under the dark condition, and the reaction formula was:
[0056]
[0057] (2) 1ml of ethylene glycol was added to the reaction solution obtained in step (1), and stirred for 5 minutes to quench the sodium periodate in the reaction solution to terminate the oxidation reaction;
[0058] (3) 200ml of ethanol with a concentration of 75% was added to the reaction solution obtained in step (2), stir...
Embodiment 3
[0067] The present embodiment is prepared according to the process steps of Example 2, and the chondroitin oxysulfate and chondroitin oxysulfate-natamycin structures generated in the preparation process are detected, analyzed and identified:
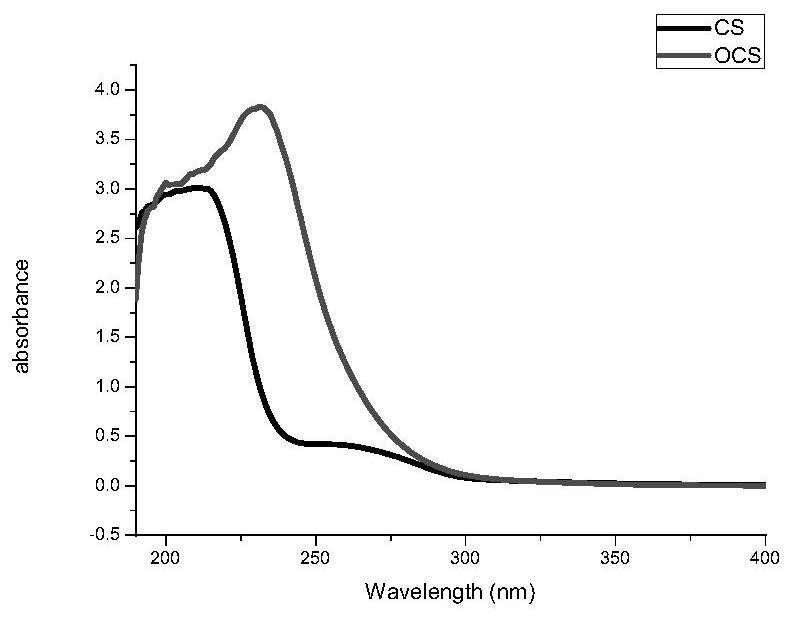
[0068] The structure of chondroitin oxysulfate was detected by ultraviolet spectrum and X-ray photoelectron spectroscopy analysis: (1) For details of ultraviolet spectrum, please refer to the accompanying drawings in the description. image 3 : Chondroitin sulfate has obvious characteristic absorption peak, the maximum absorption wavelength is at 213nm, and the characteristic absorption peak of oxidative chondroitin sulfate has obvious chemical shift, and the maximum absorption wavelength is at 231nm. It shows that the oxidation reaction is successful and a new compound is formed. (2) For details of X-ray photoelectron spectroscopy, please refer to the accompanying drawings in the description. Figure 4 : The C1s spectrum of chondroitin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com