Pyranopyrazole acrylate derivative as well as preparation method and application thereof
A technology of pyranopyrazole acrylic acid and acrylic acid ester, which is applied in the application field of pyranopyrazole acrylic acid ester derivatives and the preparation of antitumor drugs, and achieves high chemical selectivity and regioselectivity, and easy-to-obtain raw materials , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
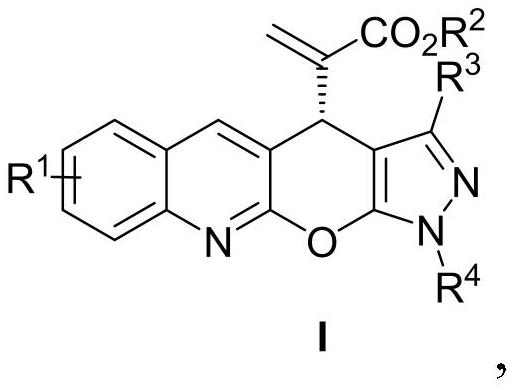
[0021] Example 1: (R)-2-(3-methyl-1-phenyl-1,4-dihydropyrazolo[4',3':5,6]pyrano[2,3-b The preparation of ] quinoline-4-yl) methyl acrylate (I-1)
[0022]
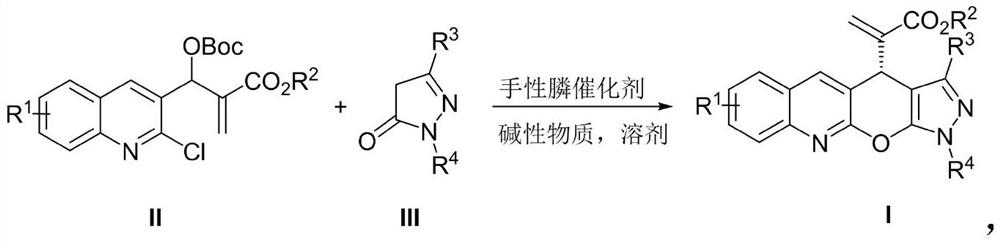
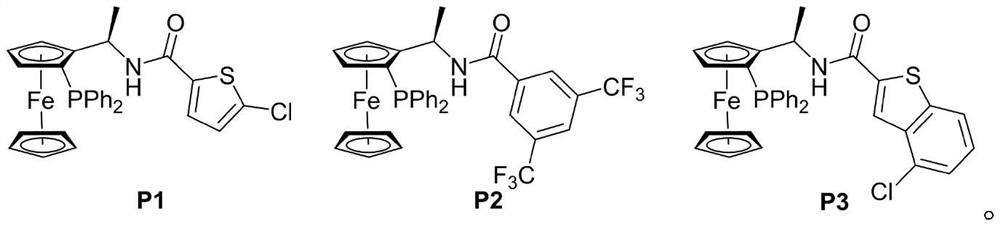
[0023] Add MBH carbonate IIa (226 mg, 0.6 mmol), pyrazolone derivative IIIa (87 mg, 0.5 mmol), sodium carbonate (53 mg, 0.5 mmol) and o-dichlorobenzene (2.5 mL) into a dry Shrek tube ( 15 mL), add chiral phosphine catalyst P3 (15 mg, 0.025 mmol), stir at 40 °C for 72 hours, concentrate under reduced pressure to recover the solvent, and separate by column chromatography (petroleum ether / ethyl acetate=3 / 1, v / v) To obtain light yellow solid I-1, 166.7mg, yield 84%, ee value 93%.
[0024] Structural characterization of I-1: m.p.: 129-130°C; 1 H NMR (400MHz, CDCl 3 )δ8.18(s,1H),7.97(d,J=8.4Hz,1H),7.91(d,J=7.6Hz,2H),7.76(d,J=8.0Hz,1H),7.71–7.66( m,1H),7.52–7.45(m,3H),7.32–7.27(m,1H),6.38(s,1H),5.87(s,1H),5.30(s,1H),3.64(s,3H) ,2.22(s,3H). 13 C NMR (100MHz, CDCl 3 )δ165.9, 155.2, 146.8, 145.9, 145.4, 142.0, 139.3, 137...
Embodiment 2
[0025] Example 2: (R)-2-(3-methyl-1-phenyl-1,4-dihydropyrazolo[4',3':5,6]pyrano[2,3-b The preparation of ] quinoline-4-yl) methyl acrylate (I-1)
[0026] Add MBH carbonate IIa (226 mg, 0.6 mmol), pyrazolone derivative IIIa (87 mg, 0.5 mmol), sodium carbonate (53 mg, 0.5 mmol) and toluene (2.5 mL) into a dry Shrek tube (15 mL) , add chiral phosphine catalyst P1 (55.7mg, 0.1mmol), stir at 25°C for 96 hours, concentrate under reduced pressure to recover the solvent, and separate by column chromatography (petroleum ether / ethyl acetate=3 / 1, v / v) , to obtain light yellow solid I-1, 99.3mg, yield 50%, ee value 98%.
Embodiment 3
[0027] Example 3: (R)-2-(3-methyl-1-phenyl-1,4-dihydropyrazolo[4',3':5,6]pyrano[2,3-b The preparation of ] quinoline-4-yl) methyl acrylate (I-1)
[0028] Add MBH carbonate IIa (226 mg, 0.6 mmol), pyrazolone derivative IIIa (87 mg, 0.5 mmol), sodium carbonate (53 mg, 0.5 mmol) and toluene (2.5 mL) into a dry Shrek tube (15 mL) , add chiral phosphine catalyst P2 (65.3mg, 0.1mmol), stir at 25°C for 96 hours, concentrate under reduced pressure to recover the solvent, and separate by column chromatography (petroleum ether / ethyl acetate=3 / 1, v / v) , to obtain light yellow solid I-1, 109.2mg, yield 55%, ee value 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com