Method for catalytically synthesizing sucrose fatty acid ester by lipase in organic solvent
A technology for synthesizing fatty acids and organic solvents, which is applied in the field of synthesizing fatty acid sucrose esters catalyzed by lipase, which can solve the problems of high production cost and production equipment, reduced production, high toxicity of strong polar organic solvents, etc., to reduce production costs and ensure safety The effect of improving performance and enhancing catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
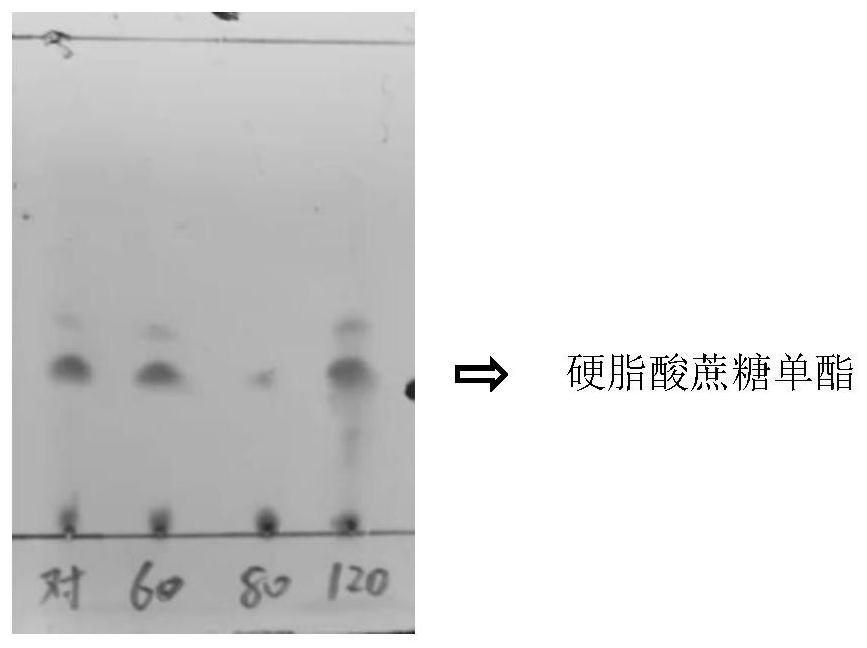
[0025] Grind the sucrose into fine powder and dry it in a vacuum oven at 60°C for 6 hours before use. 0.1 mmol (0.034 g) of sucrose, 2.25 mmol (585 μL) of vinyl laurate, and 15 mL of tert-amyl alcohol or tert-butanol were added to two 50 mL round-bottomed flasks, respectively, and stirred for 1 h in a 60° C. water bath. Then add 0.1g Novozym435, 0.09g sodium laurate and 0.4g respectively Molecular sieves were reacted in a water bath at 60°C for 24h. After the reaction, the reaction solution was filtered to recover Novozym435, then washed with tert-amyl alcohol, and dried in vacuum for use. The sucrose monoester concentration in the tert-amyl alcohol system was 2.32 g / L, and the yield was 66.9% by high performance liquid chromatography; the sucrose monoester concentration in the tert-butanol system was 2.04 g / L, and the yield was 58.8%.
[0026] It can be seen that the yield of tert-amyl alcohol system is higher than that of tert-butanol system, so tert-amyl alcohol is used ...
Embodiment 2
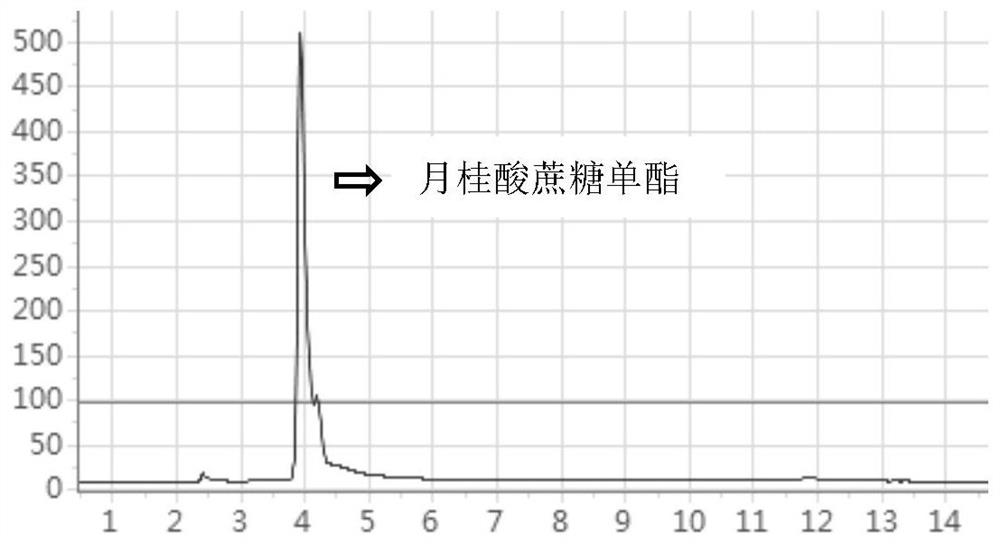
[0028] Grind the sucrose into fine powder and dry it in a vacuum oven at 60°C for 6 hours before use. Add 0.08 / 0.1 / 0.4 / 0.6 / 0.8 mmol of sucrose, 2.25 mmol (585 μL) of vinyl laurate and 15 mL of tert-amyl alcohol to five 50 mL round-bottomed flasks, respectively, and stir in a 60° C. water bath for 1 h. Then add 0.1g Novozyme435, 0.09g sodium laurate and 0.4g respectively to the mixture. Molecular sieves were reacted in a water bath at 60°C for 24h. After the reaction, the reaction solution was filtered to recover Novozym435, then washed with tert-amyl alcohol, and dried in vacuum for use. The sucrose monoester concentrations in the tert-amyl alcohol system were measured by high performance liquid chromatography to be 2.3 / 3.61 / 4.26 / 4.28 / 4.13 g / L, respectively.
[0029] It can be seen that the yield of sucrose monoester increases with the increase of sucrose concentration, but when the sucrose concentration in the system is greater than 0.6mmol / 15mL, the yield of sucrose monoe...
Embodiment 3
[0031] Grind the sucrose into fine powder and dry it in a vacuum oven at 60°C for 6 hours before use. 1.51 / 1.70 / 2.25 / 3.40 / 3.61 mmol of vinyl laurate, 0.4 mmol (0.137 g) of sucrose and 15 mL of tert-amyl alcohol were added to five 50 mL round-bottomed flasks, and the mixture was stirred in a 60° C. water bath for 1 h. Add 0.1g Novozyme435, 0.09g sodium laurate and 0.4g respectively to the mixture Molecular sieves were reacted in a water bath at 75°C for 24h. After the reaction, the reaction solution was filtered to recover Novozym435, then washed with tert-amyl alcohol, and dried in vacuum for use. The sucrose monoester concentrations in the tert-amyl alcohol system were measured by high performance liquid chromatography to be 4.86 / 5.40 / 6.42 / 6.40 / 6.37 g / L, respectively.
[0032] It can be seen that the production of sucrose monoester increases with the increase of the concentration of vinyl laurate, but when the concentration of vinyl laurate in the system is greater than 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com