Alginate lyase mutant capable of relieving dependence of divalent metal ions and application of alginate lyase mutant
A technology of alginate lyase and divalent metal ions, which is applied in the field of protein engineering and can solve problems such as limiting the wide use of alginate lyase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
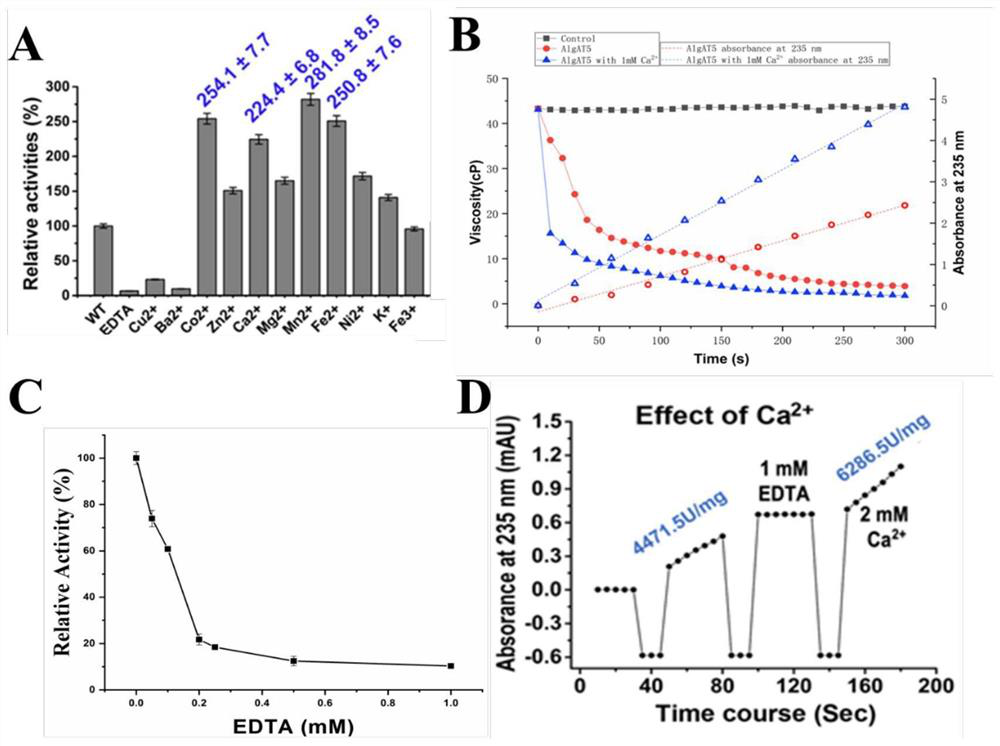
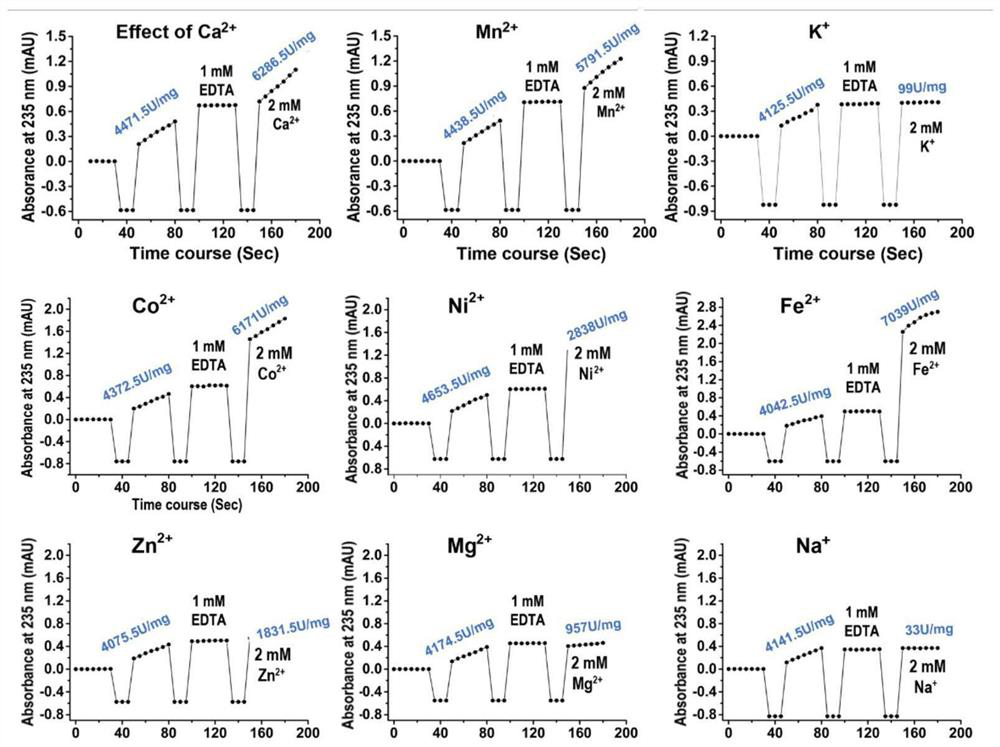
[0032] Example 1. Analysis of metal ion-dependent alginate lyases of the PL7 family based on structure and catalytic properties
[0033] After analyzing the alginate lyases in the PL7 family, we found that most of the enzymes whose substrate specificity is PolyG have obvious divalent metal ion activation effects, and the addition of these divalent metal ions will significantly increase their enzyme activity. However, adding After metal ion chelating agents such as EDTA or EGTA remove these divalent metal ions in the reaction system, the enzyme activity is significantly reduced.
[0034] Take alyPG as an example, add Zn 2+ and Mn 2+ Afterwards, compared with the wild type, the enzyme activity increased to 131% and 175%, but after adding the metal ion chelating agent EDTA, the enzyme activity decreased to 17% of the wild type. For Alg_M3, Ca 2+ mn 2+ 、Co 2 + , Mg 2+ Can significantly increase enzyme activity; for Alg, Ca 2+ , Mn 2+ Can significantly increase the enzyme...
Embodiment 2
[0040] Embodiment 2, the induced expression of AlgAT5 in Escherichia coli
[0041] The expression plasmid containing pET-30a-AlgAT5 stored in a -80°C refrigerator [Qingdao Institute of Bioenergy and Biotechnology, Chinese Academy of Sciences, Li Fuli, Su Hang, Ji Shiqi, Lu Ming. The gene encoding alginate lyase and its application: China , 201810862414.3[P].2018-08-01.] BL21 protein expression strain, 5 μL was taken out and inoculated in 5 mL of LB plus kanamycin liquid medium at 200 rpm, and cultivated overnight at 37°C as seeds. The next day, transfer the activated cells to 500mL LB liquid medium containing kanamycin, culture at 200rpm at 37°C until the OD600 is 0.5-0.8, add IPTG with a final concentration of 1mM, and place at 22-25°C The culture was shaken at 200rpm in a constant temperature shaker for 16-18h to induce protein expression. Alginate lyase AlgAT5 was purified with Ni-NTA Resin (purchased from TransGen Biotech). After the purification was completed, the purif...
Embodiment 3
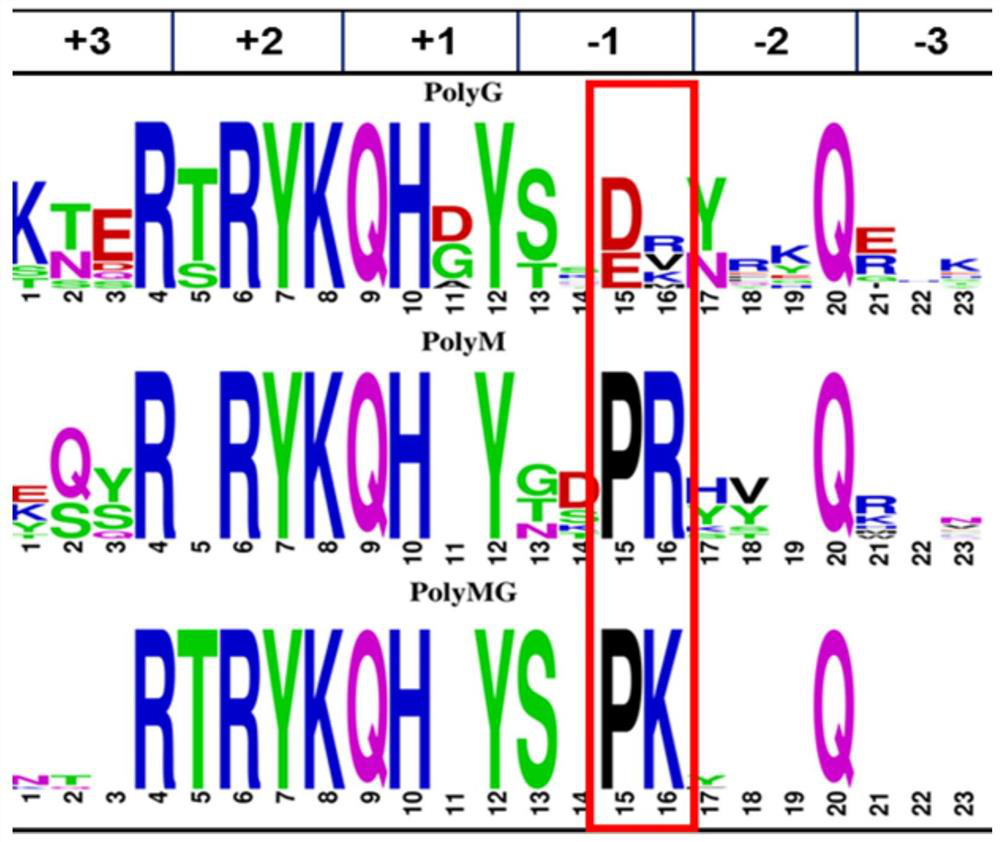
[0052] Example 3. Construction and enzyme activity determination of D146E, D146H, D146K, D146R, D146A, D146P mutants.
[0053] Based on the analysis in Example 2, a site-directed mutation was performed on the amino acid at position 146, and further analysis of the amino acids near the substrate binding region in the PL7 family, combined with the results of multiple sequence alignments with different substrate specificities, we found that Asp146 is the key factor that determines PolyG and The key amino acid of PolyM substrate specificity, we speculate that Asp146 also plays an important role in the binding of metal ions.
[0054] According to the properties of amino acids, it can be known that aspartic acid D is an amino acid with a negative charge on the side chain, and glutamic acid E also has this property, so it is preferred to mutate it into one with the same negative charge but a slightly longer side chain length Glutamate E. And further choose to mutate it into positive...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com