D-A type conjugated polymer as well as preparation method and application thereof
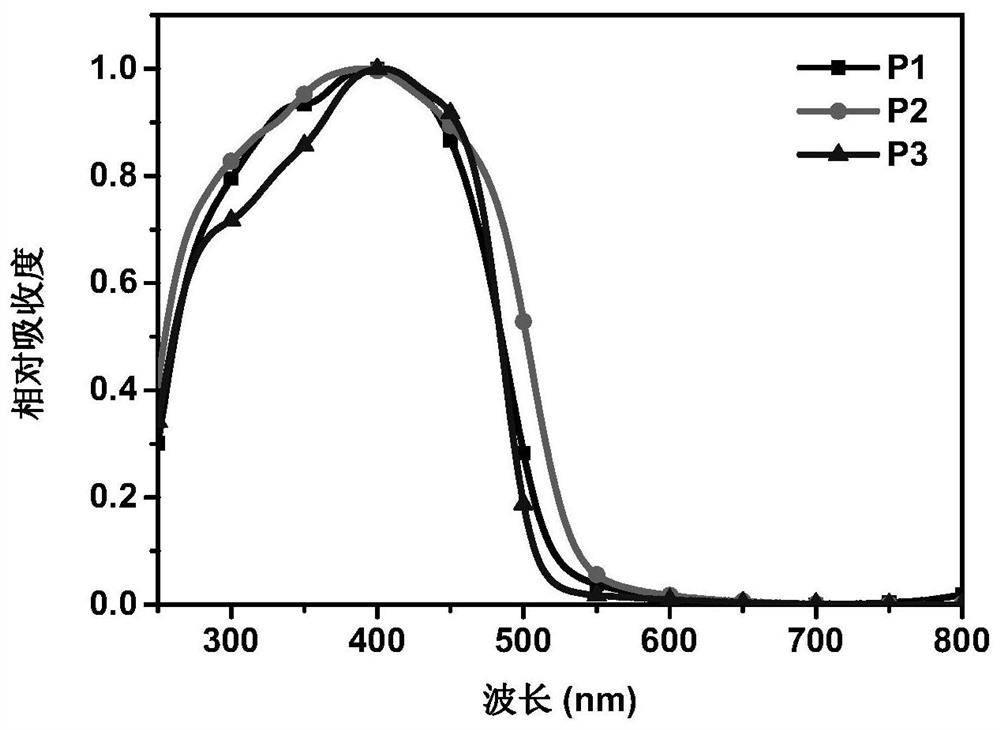
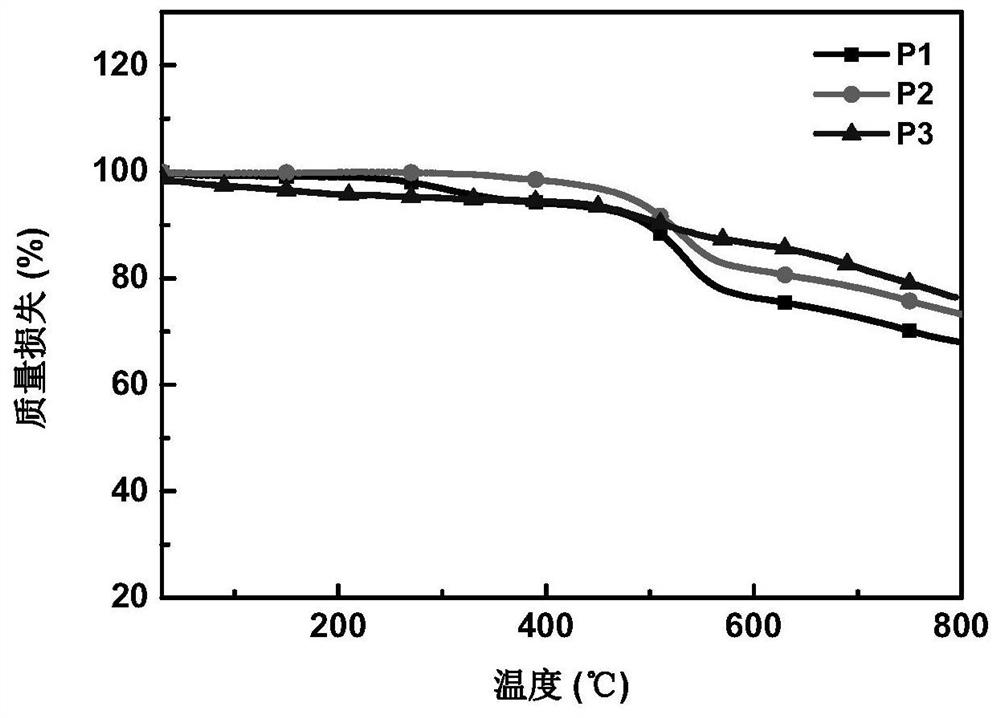
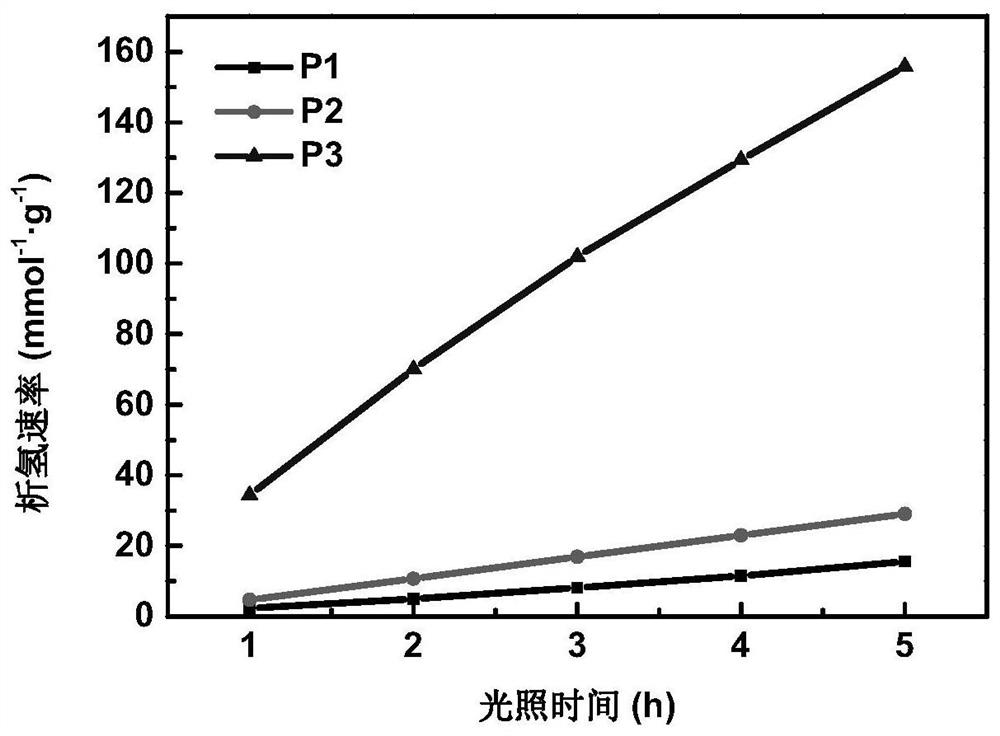
A conjugated polymer, D-A technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve the problems of low hydrogen evolution rate and high hydrogen evolution rate, and achieve strong Absorption, high chemical and thermal stability, and efficient photocatalytic water splitting for hydrogen production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthetic route of D-A type conjugated polymer described in this embodiment is as follows:
[0033]
[0034] The preparation method of D-A type conjugated polymer described in this embodiment is as follows:
[0035] (1) Synthesis of monomer M1:
[0036] Weigh dibenzothiophene sulfone (10mmol, 2.1625g) into a 25mL round-bottomed flask, add 70mL of concentrated sulfuric acid, stir well, and add NBS (21mmol, 3.7380g) in batches at 0°C; After reacting for 24h, the reaction solution was poured into ice water and stirred, the solid was washed with water and methanol until PH = 7, passed through CH 3 Cl recrystallization gave 3,7-dibromodibenzo[b,d]thiophene 5,5-dioxide (white solid);
[0037] Weigh 3,7-dibromodibenzo[b,d]thiophene 5,5-dioxide (10mmol, 3.7405g), biboronic acid pinacol ester (30mmol, 7.620g), potassium acetate (60mmol, 5.880 g) and Pd(dppf)Cl 2 (0.5mmol, 0.3658g) was added to a 250mL two-necked flask, and the gas was ventilated three times to remove ...
Embodiment 2
[0042] The synthetic route of D-A type conjugated polymer described in this embodiment is as follows:
[0043]
[0044] The preparation method of D-A type conjugated polymer described in this embodiment is as follows:
[0045] (1) Synthesis of conjugated polymer P2;
[0046] Add monomer M1 (0.5mmol, 0.2341g) and monomer M3 (0.25mmol, 0.1139g) in a 48ml glass pressure bottle; then add Pd (pph) in a nitrogen-filled glove box 3 ) 4 (0.025mmol, 29mg), K 2 CO 3(2mol / L, 6mL) and DMF (12mL), tighten the tetrafluoro stopper; react at 160°C for 48h. After the reaction solution was cooled to room temperature, it was dropped into methanol solution, and the crude product was obtained by filtration, which was subjected to Soxhlet extraction with methanol, n-hexane, dichloromethane, and tetrahydrofuran, respectively. The remaining solid was washed with methanol and dried in vacuum for 24 hours to obtain 152.7 mg of yellow-brown conjugated polymer P2. After testing, the degree of po...
Embodiment 3
[0049] The synthetic route of D-A type conjugated polymer described in this embodiment is as follows:
[0050]
[0051] The preparation method of D-A type conjugated polymer described in this embodiment is as follows:
[0052] (1) Synthesis of monomer M5:
[0053] Weigh 1,3,5-tribromobenzene (10mmol, 3.1480g), thiophen-2-ylboronic acid (35mmol, 4.4786g) and Pd (pph 3 ) 4 (0.5mmol, 0.5778g) was added into a 250mL two-necked flask, and the oxygen in the reaction flask was removed by pumping and purging three times. Add potassium phosphate (2mmol·L -1 , 10 mL) and anhydrous THF (100 mL). React at 85°C for 20h. Washed with saturated NaCl solution and extracted with dichloromethane, the crude product was purified by silica gel column chromatography, and recrystallized with tetrahydrofuran / methanol to obtain white solid 1,3,5-tri(thiophen-2-yl)benzene;
[0054] Weigh 1,3,5-tris(thiophen-2-yl)benzene (1mmol, 0.3245g) into a round bottom flask, add THF (20mL); at 0°C, add NBS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com