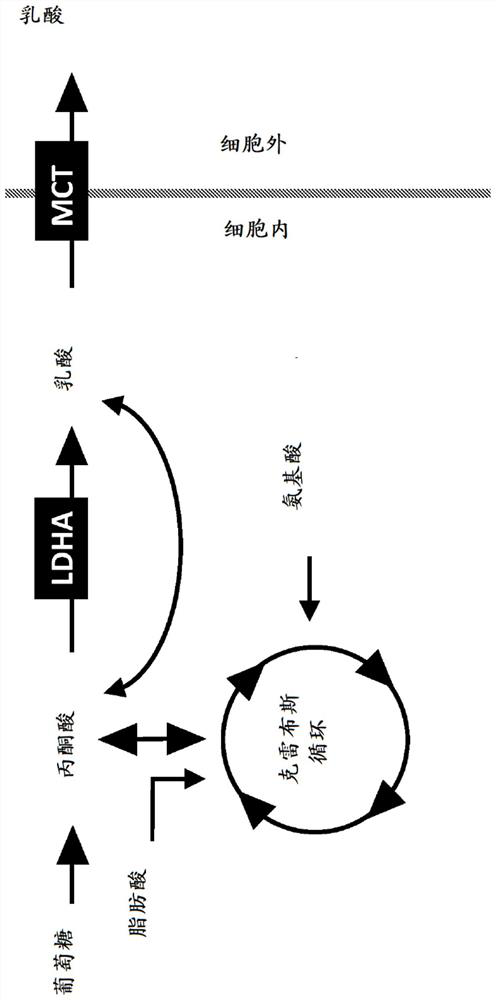
Chimeric receptor polypeptides in combination with trans metabolism molecules modulating intracellular lactate concentrations and therapeutic uses thereof
A chimeric receptor, regulatory factor technology, used in fusion polypeptides, for targeting specific cell fusion, growth factors/growth regulators, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0129] In other embodiments, the extracellular antigen binding domain of any of the CAR polypeptides described herein can be specific for a pathogenic antigen, such as a bacterial, viral, or fungal antigen. Some examples are provided below: influenza virus neuraminidase, hemagglutinin or M2 protein, human respiratory syncytial virus (RSV) F or G glycoprotein, herpes simplex virus glycoprotein gB, gC, gD or gE, Chlamydia MOMP or PorB protein, dengue virus core protein, matrix protein or glycoprotein E, measles virus hemagglutinin, herpes simplex virus type 2 glycoprotein gB, poliovirus I VP1, envelope glycoprotein of HIV 1, B Hepatitis core antigen or surface antigen, diphtheria toxin, streptococcal 24M epitope, gonococcal pili protein, pseudorabies virus g50 (gpD), pseudorabies virus II (gpB), pseudorabies virus III (gpC), pseudorabies virus sugar Protein H, pseudorabies virus glycoprotein E, transmissible gastroenteritis glycoprotein 195, transmissible gastroenteritis matrix ...
Embodiment 1
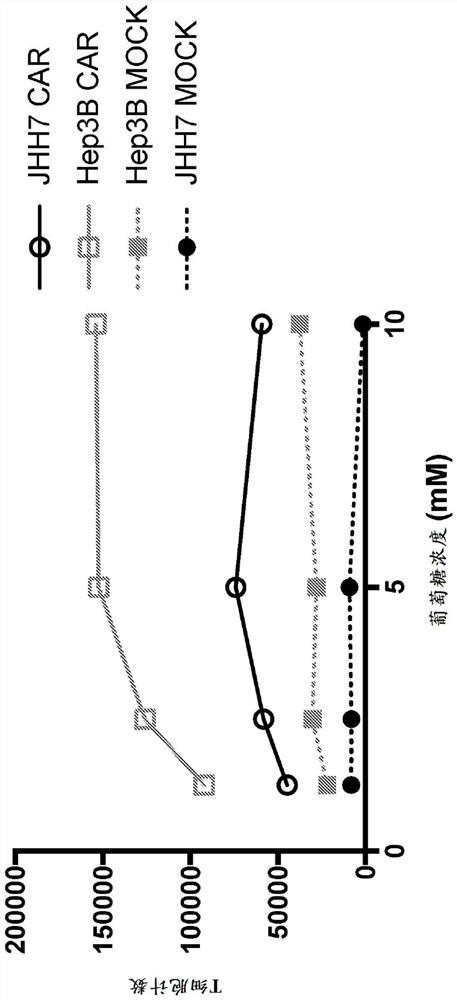
[0494] Example 1: Effect of reduced glucose concentration on T cell function
[0495] A γ-retrovirus encoding an exemplary GPC3-targeting CAR expression construct of SEQ ID NO: 97 was generated by recombinant technology and used to infect primary human T cells to produce cells expressing the target on their cell surface. cells to the CAR polypeptide of GPC3. A six-day flow-based proliferation assay was then used to test the functionality of CAR-expressing cells targeting GPC3. Specifically, 200,000 non-transduced mock T cells or T cells expressing a CAR construct targeting GPC3 were mixed with 50,000 GPC3+ hepatocellular carcinoma JHH7 tumor cells or Hep3B tumor cells at a ratio of 4:1 (effector cells / CAR-expressing The ratio of T cells to target cells) was incubated together. In the presence of different concentrations of glucose, the co-culture was incubated at 37 °C in 5% CO 2 Incubate for six days in the incubator. At the end of six days, co-cultures were harvested and...
Embodiment 2
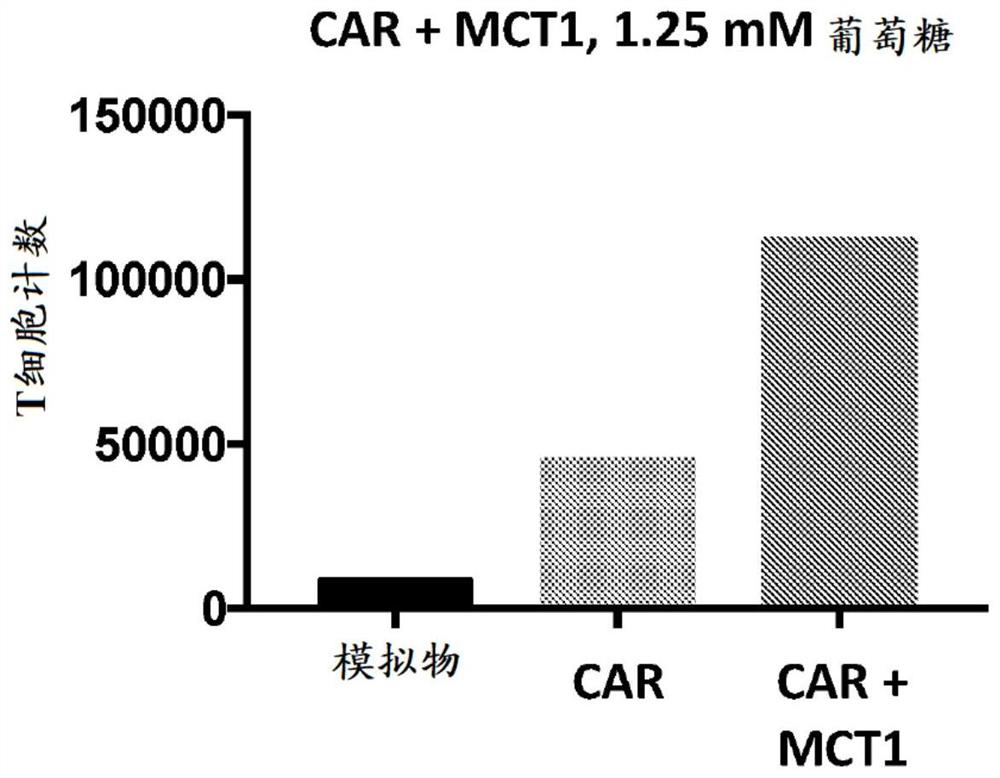
[0496] Example 2: Effect of expressing lactate regulatory factors on T cell function when using GPC3-targeting CAR-T expression constructs
[0497]A gamma retrovirus encoding an exemplary GPC3-targeting CAR polypeptide expression construct (SEQ ID NO:97) was produced by recombinant technology and used to infect primary human T cells to produce Cells expressing CAR polypeptides targeting GPC3. In addition, gamma coding for exemplary GPC3-targeting CAR polypeptides (SEQ ID NO:97 or 98) and lactate transporting polypeptides (MCT1, MCT2 or MCT4) (SEQ ID NO:82 to SEQ ID NO:84) were produced by recombinant technology A retroviral vector was used to infect primary human T cells to generate cells expressing a GPC3-targeting polypeptide and a lactate-regulating factor (eg, polypeptide). In constructs encoding both the CAR polypeptide and the lactate regulator, the two polypeptides are separated by a P2A ribosomal skipping sequence. The variants expressed are CARs in combination with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com