Quinone reductase 2 inhibitor compounds and uses thereof
A compound and pharmaceutical technology, applied in the field of quinone reductase 2 inhibitor compound and its use, can solve problems such as limitations, efficacy and side effects, bull's eye macular degeneration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Example 1: Development of non-lysosomal aminoquinoline inhibitors of QR2.
[0186] Chloroquine and hydroxychloroquine are lysosomotropic drugs that preferentially accumulate in cellular lysosomes. For chloroquine, the pKa of the tertiary amine nitrogen is 10.32, while the pKa of the quinoline nitrogen is 7.29. At acidic lysosomal pH between 4-5.5, nearly 100% of chloroquine is doubly protonated, giving the molecule a 2+ charge, making it strongly hydrophilic, membrane-impermeable, and thus trapped in Acidic organelles.
[0187] A quantitative treatment of this trapping phenomenon can be obtained by examining the octanol-water partition coefficient log D of a drug, which describes the relative partition properties of all forms of a compound at different pH. For a given pH, compounds with positive logD are relatively lipophilic and more membrane permeable, while compounds with negative logD are hydrophilic and less membrane permeable.
[0188] exist Figures 1A-1B The...
Embodiment 2
[0194] Example 2: QR2 Inhibitor Synthesis and Characterization.
[0195]
[0196] A suspension of 4,7-dichloroquinoline (2.0 g, 10.2 mmol) in aqueous methylamine (40% 20 mL 260 mmol, 26 equiv) was heated in a microwave vessel at 90 °C (initial power setting 150 W) 2 hours. by TLC (2% MeOH / CH 2 Cl 2 ) analysis of the reaction mixture indicated complete consumption of starting material. The reaction mixture was treated with H 2 O (100 mL) was diluted, and the insoluble material was collected under vacuum. filter cake with H 2 O washed and dried in vacuo to give the pure product as a white microcrystalline solid (1.8 g, 92%). 1 H NMR (DMSO- d 6 , 300 MHz) δ 8.40 (d, J = 5.1 Hz, 1H), 8.16 (d, J = 9.0 Hz, 1H), 7.77(s, 1H), 6.38 (d, J = 5.4 Hz, 1H), 2.86 ( d, J = 5.4 Hz, 3H). ESIMS: m / z = 193[(M+H) + ].
[0197]
[0198] General procedure for 7-substituted-4-(pyridin-3-yl)-methylaminoquinolines. A mixture of 7-substituted-4-chloroquinoline (5.1 mmol), 3-aminome...
Embodiment 3
[0203] Example 3: Comparative testing of QR2 inhibitors.
[0204]
[0205] Table 1: Comparative ICs 50 data.
[0206] IC 50 (µM)
standard deviation
QR2 I-44 7.51 0.66 Compound 1 7.23 1.51 Compound 2 15.50 4.84 Chloroquine 70.92 5.27
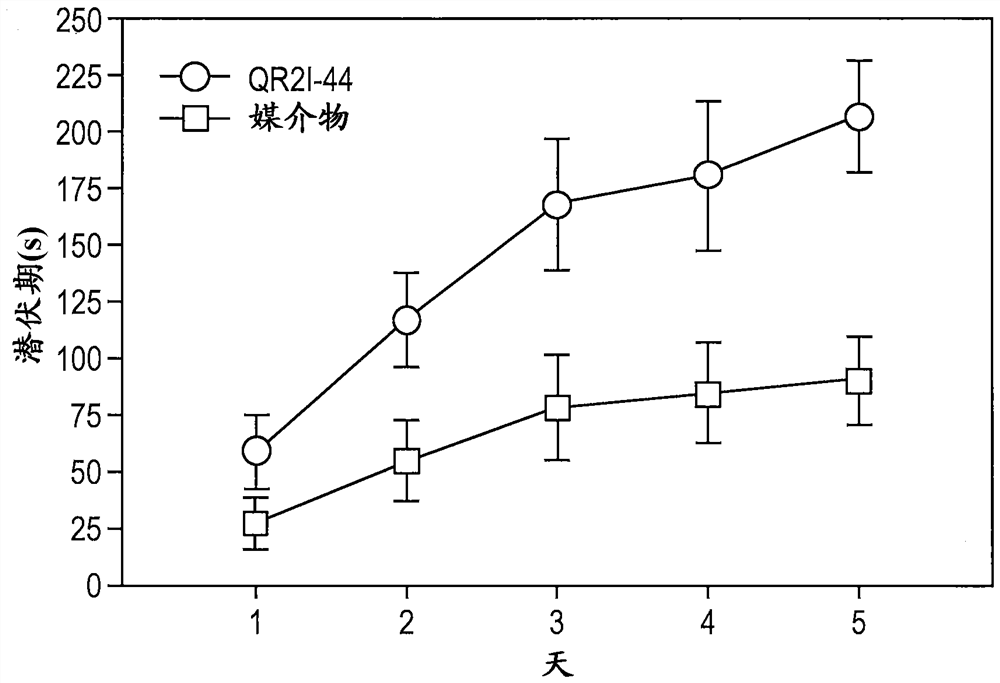
[0207] We investigated the neuroprotective potential of QR2I-44 in a closed head injury (TBI) mouse model (Laskowitz et al., “Neuroprotective pentapeptide CN-105 is associated with reduced sterile inflammation and improved functional outcomes in a traumaticbrain injury murine model” , Sci. Rep. 2017 Apr 21;7:46461; Laskowitz et al., “Traumatic brain injury exacerbates neurodegenerative pathology: improvement with an apolipoprotein E-based therapeutic.” Journal of Neurotrauma 2010m 27:1983-1995). The closed head impact model results in damage to selectively vulnerable neurons in the cortex, basal ganglia, and hippocampus, induces diffuse axonal damage, and results in measurable vestibular ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com