A large-scale qualitative method for glycosides based on liquid chromatography-mass spectrometry
A liquid chromatography and compound technology, which is applied in the field of large-scale characterization of glycoside compounds, can solve the problems of high price, limited glycoside compounds, limited scale of glycoside compound secondary mass spectrometry database, etc., to avoid redundancy and high reliability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
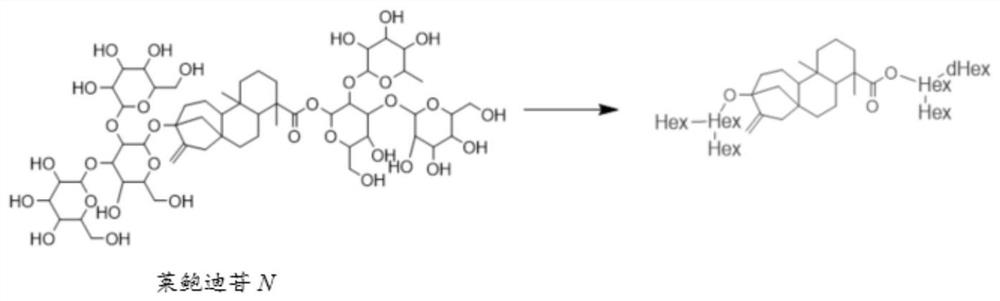
[0034] The identification of embodiment 1 stevioside-rebaudioside N
[0035] The technical scheme that the present invention adopts is as follows:
[0036] 1. Sample configuration: the commercially available rebaudioside N standard was dissolved in pure methanol, and the sample concentration was 50ng / mL.
[0037] 2. Instrument analysis conditions:
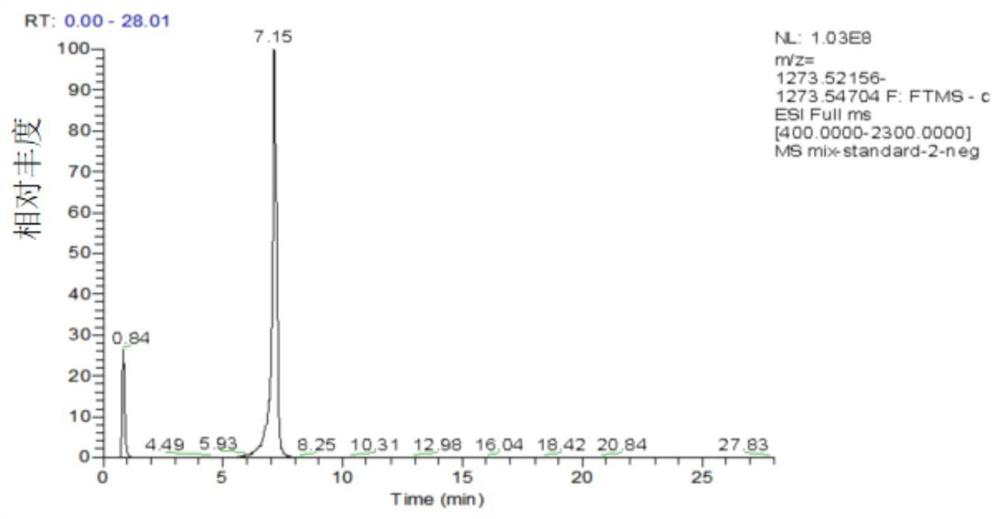
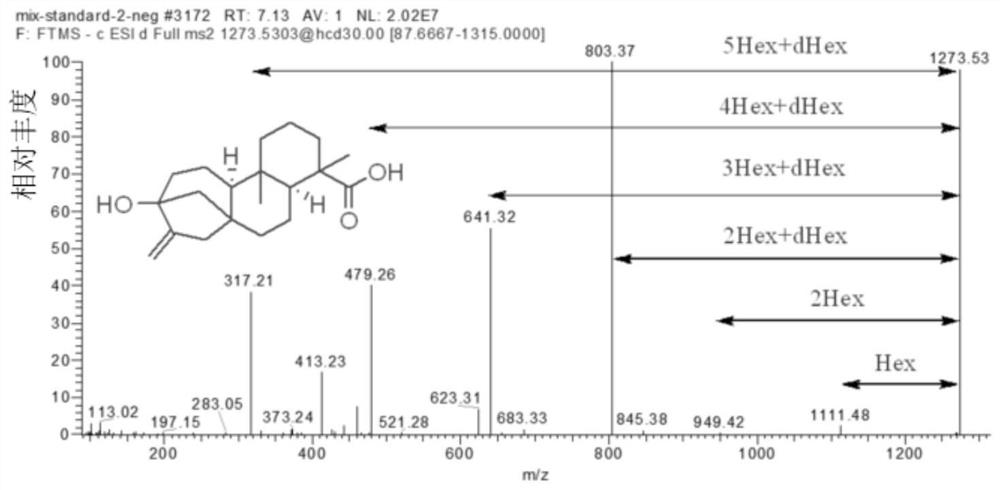
[0038]Chromatographic conditions: ACQUITY UHPLC ultra-high performance liquid chromatography system (UPLC, Waters, Milford, MA, USA.) and Q Exactive HF high-resolution mass spectrometry (Thermo Fisher Scientific, Rockford, IL, USA.) were used as analytical instruments. The liquid chromatography conditions are: phase A and phase B are respectively 0.1% formic acid / water (volume ratio) and 0.1% formic acid / acetonitrile (volume ratio). The flow rate was 0.1 mL / min. The total analysis time is 28min. The elution gradient started with 10% B (volume ratio) and was held for 2 min. Then increase linearly to 100% B within 22min and keep...
Embodiment 2
[0056] Example 2 Large-scale characterization of glycosides in the crude stevia extract.
[0057] The technical solution is as follows:
[0058] 1. The sample extraction method is: accurately weigh 5 mg of stevia dry powder into a 1.5 mL centrifuge tube, add 1 mL of ethanol / water solution (volume ratio 50:50) to extract for 2 hours, shake on a vortex shaker for 1 minute, and place in a high-speed centrifuge Centrifuge at 4°C and 15,000rpm for 10min, take the supernatant and freeze-dry, add 1mL of methanol / water (volume ratio, 8:2) to the freeze-dried sample powder, and centrifuge at 4°C at 15,000rpm in a high-speed centrifuge. After 10 minutes, the supernatant was taken for analysis.
[0059] 2. Instrument analysis conditions: with embodiment 1.
[0060] 3. Stevioside aglycon library construction: same as Example 1.
[0061] 4. Glycosyl theoretical characteristic neutral loss library construction: Same as Example 1.
[0062] 5. Identification of glycosides in crude stevia ...
Embodiment 3
[0075] Example 3 Scale Identification of Glycoside Compounds of Corn Leaf Crude Extract
[0076] 1. Preparation method of corn leaf crude extract: Accurately weigh 50mg of corn leaf powder into a 1.5mL centrifuge tube, add 1mL of 80% methanol / water solution and vortex extract for 5min, centrifuge at 4°C and 15000rpm for 10min at high speed, and take 700μL The supernatant was lyophilized overnight, redissolved in 100 μL of 80% methanol / water, centrifuged again at 15,000 rpm for 10 min at 4°C, and the supernatant was taken for analysis.
[0077] 2. Instrument analysis conditions:
[0078] The analytical instrument used for the non-targeted LC-MS / MS metabolome data acquisition is the same as that in Example 1. In the positive ion mode, the liquid chromatography conditions are: phase A and phase B are respectively 0.1% formic acid / water (volume ratio) and 0.1% formic acid / acetonitrile (volume ratio). The flow rate was 0.35 mL / min. The total analysis time is 30 min. The elution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com