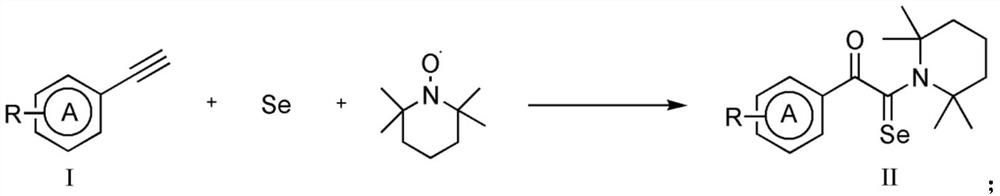
Synthesis method of alpha-oxo-selenoamide derivative
A synthetic method and technology of selenoamide, applied in the direction of organic chemistry, etc., can solve problems such as poor economy, harsh reaction conditions, and high input costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of 1-phenyl-2-(2,2,6,6-tetramethylpiperidinyl)-2-selenoacetamide (20)
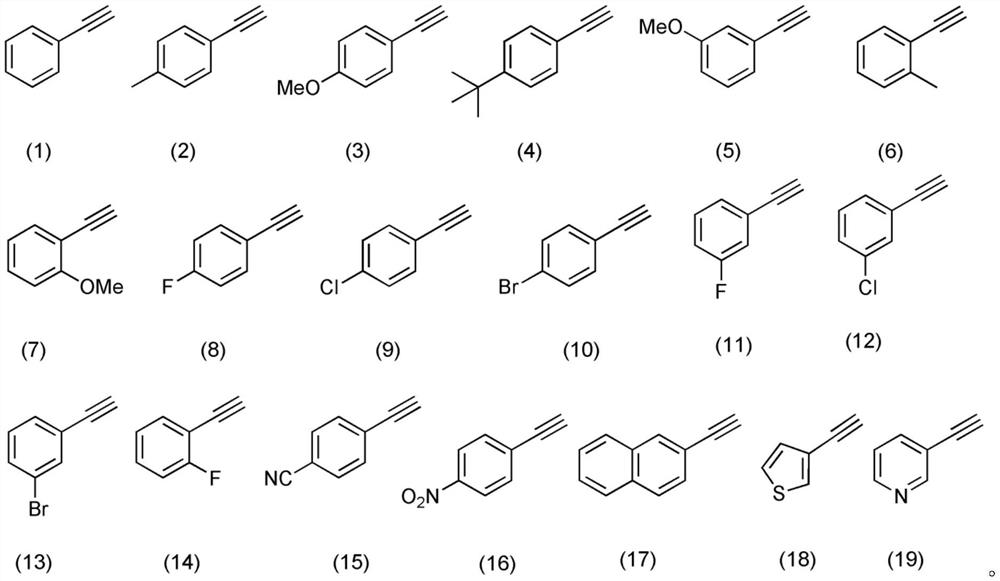
[0040] Weigh 0.9mmol phenylacetylene (compound corresponding to number (1), 0.0919g), 0.9mmol selenium powder (0.0710g), 0.3mmol 2,2,6,6-tetramethylpiperidine oxide (0.0468g), Add 0.9mmol of silver fluoride (0.1141g) to an 8mL reaction flask, add 3mL of N,N-dimethylacetamide as a solvent, stir and react at 50°C for 10 hours; Chromatographic separation (column chromatography separation conditions: the stationary phase is 200-300 mesh silica gel powder, the mobile phase is ethyl acetate (A) and petroleum ether (B), and the mobile phase change program (A:B) is 1:20) , to obtain 0.0787 g of the reaction product.
[0041] The above-mentioned reaction product is characterized, and the result is: 1 H NMR (400MHz, deuterated chloroform) δ8.06-8.03(m,2H),7.96-7.36(m,3H),2.35(s,1H),2.15(s,6H),1.90-1.85(m,3H) ), 1.65(s,3H), 1.51(s,2H), 1.33(s,3H)ppm; according to the characterization data, the rea...
Embodiment 2
[0043] Synthesis of 1-(4-methylphenyl)-2-(2,2,6,6-tetramethylpiperidinyl)-2-selenoacetamide (21)
[0044] Weigh 0.9mmol 4-methylphenylacetylene (compound corresponding to number (2), 0.1045g), 0.9mmol selenium powder (0.0710g), 0.3mmol 2,2,6,6-tetramethylpiperidine oxide (0.0468g), 0.9mmol silver fluoride (0.1141g) in an 8mL reaction flask, add 3mL of N,N-dimethylacetamide as a solvent, stir and react at 50°C for 10 hours; After vacuum concentration and column chromatography separation (column chromatography separation conditions: the stationary phase is 200-300 mesh silica gel powder, the mobile phase is ethyl acetate (A) and petroleum ether (B), and the mobile phase change program (A:B) 1:20), to obtain 0.0505g reaction product.
[0045] The above-mentioned reaction product is characterized, and the result is: 1 H NMR (400MHz, deuterated chloroform) δ = 7.94 (d, J = 8.0Hz, 2H), 7.19 (d, J = 8.0Hz, 2H), 2.38 (s, 3H), 2.15 (s, 6H), 1.89 -1.85(m,3H), 1.65(s,3H), 1.33(s,3H), 1....
Embodiment 3
[0047] Synthesis of 1-(4-bromophenyl)-2-(2,2,6,6-tetramethylpiperidinyl)-2-selenoacetamide (29)
[0048] Weigh 0.9mmol 4-bromophenylacetylene (compound corresponding to numbering (10), 0.1629g), 0.9mmol selenium powder (0.0710g), 0.3mmol 2,2,6,6-tetramethylpiperidine oxide (0.0468g), 0.9mmol silver fluoride (0.1141g) in an 8mL reaction flask, add 3mL of N,N-dimethylacetamide as a solvent, stir and react at 50°C for 10 hours; After vacuum concentration and column chromatography separation (column chromatography separation conditions: the stationary phase is 200-300 mesh silica gel powder, the mobile phase is ethyl acetate (A) and petroleum ether (B), and the mobile phase change program (A:B) was 1:20), and 0.0509 g of the reaction product was obtained.
[0049] The above-mentioned reaction product is characterized, and the result is: 1 H NMR (400MHz, deuterated chloroform) δ = 7.92 (d, J = 12.0Hz, 2H), 7.52 (d, J = 8.0Hz, 2H), 2.37 (s, 1H), 2.12 (s, 6H), 1.91 -1.84 (s, 3H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com