Method for detecting peramivir intermediate I by reversed-phase high performance liquid chromatography
A reversed-phase high-performance liquid phase, chromatographic detection technology, applied in the field of reversed-phase high-performance liquid chromatography detection of peramivir trihydrate intermediate I, can solve the problems affecting the quality of peramivir and drug safety, quality differences Large size, difficulty in mobile phase pH screening, etc., to achieve the effects of short detection time, accurate detection and quality control, and guaranteed specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
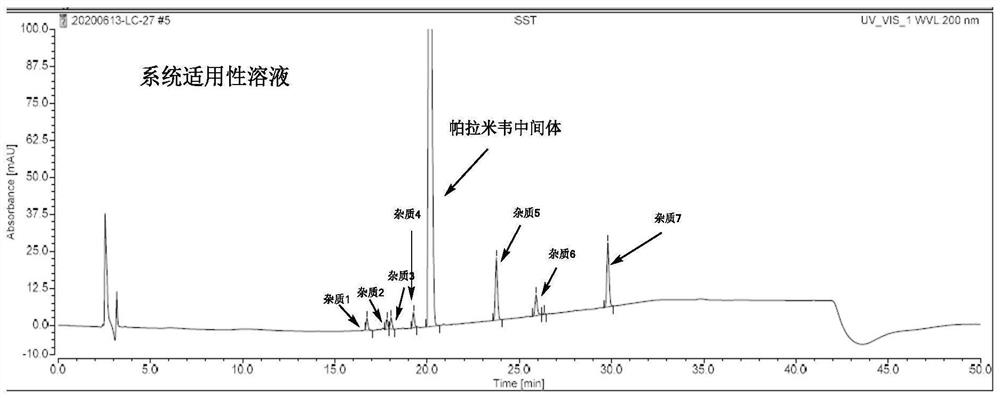
[0038] Embodiment 1: specificity test
[0040] Impurity 1 positioning solution: Take about 5 mg of the impurity 1 reference substance, weigh it accurately, put it in a 10ml measuring bottle, add diluent to dissolve and dilute to the mark and shake well to prepare a solution with a concentration of the impurity 1 reference substance of about 0.5 mg / ml.
[0041] Impurity 2 positioning solution: take about 5 mg of the impurity 2 reference substance, weigh it accurately, put it in a 10ml measuring bottle, add diluent to dissolve and dilute to the mark and shake well to prepare a solution with a concentration of the impurity 2 reference substance of about 0.5 mg / ml.
[0042] Impurity 3 positioning solution: Take about 5 mg of the impurity 3 reference substance, weigh it accurately, put it in a 10ml measuring bottle, add diluent to dissolve and dilute to the mark and shake well to prepare a solution with a concentration of the impurity 3 reference substanc...
Embodiment 2
[0055] Embodiment 2: sensitivity test
[0056] Take the system suitability solution prepared in Example 1 and dilute it step by step to an appropriate multiple. The solution with a signal-to-noise ratio ≥ 10:1 is used as the solution for the limit of quantification; the solution with a signal-to-noise ratio ≥ 3: 1 is used as the solution for the limit of detection.
[0057] Precisely measure 5 μl of each of the above solutions, inject them into the liquid chromatograph, continuously inject 6 injections of the limit of quantification solution, inject 1 injection of the detection limit solution, and record the chromatogram. The results are shown in Table 2.
[0058] Table 6 Quantitative limit, detection limit result
[0059]
[0060]
Embodiment 3
[0061] Embodiment 3: linearity test
[0062] Get the peramivir intermediate reference substance solution and dilute it with a diluent to make a series of concentration control solutions, inject it into the liquid chromatograph, record the chromatogram, and the results are shown in Table 7.
[0063] Table 7 linear experiment results
[0064]
[0065] It can be seen from Table 7 that within the range of 3.11-15.54 μg / ml, the detection method of the present invention has a good linear relationship.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com