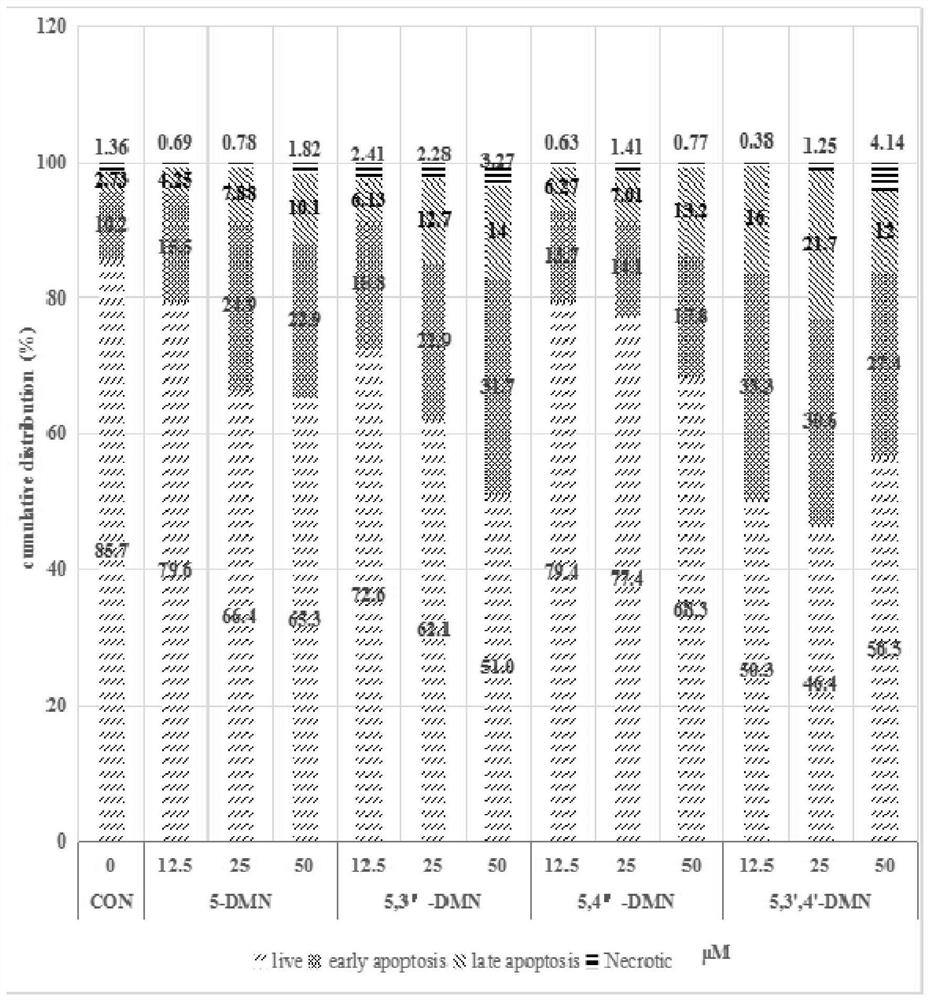
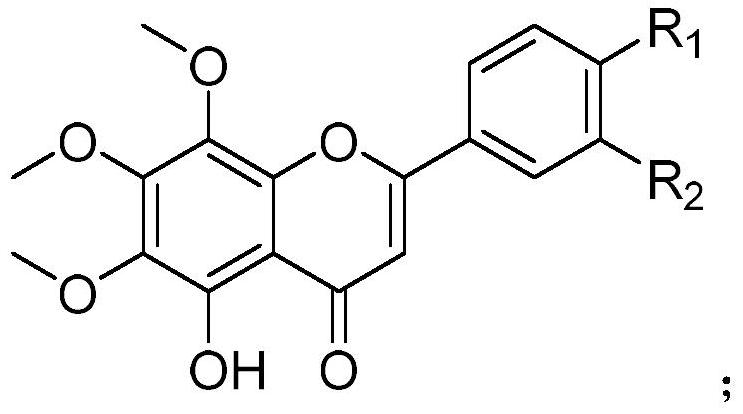
Hydroxylated polymethoxylated flavone and preparation method thereof
A polymethoxyl flavonoid, hydroxylation technology, applied in the direction of organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problem that the anti-liver cancer activity has not been reported in the literature, and achieve simple preparation method, good anti-proliferation activity, good The effect of pro-apoptotic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This embodiment provides the synthesis method of the synthesis of target product 5-DMN, and the synthetic reaction of target product 5-DMN is as follows:
[0032] Dissolve 1 mmol of nobiletin in 25 mL of anhydrous acetonitrile, add 8 mmol of anhydrous aluminum chloride, stir at 60° C. for 3 h, and detect the end point of the reaction by TLC.
[0033] Add 20mL of 3% hydrochloric acid solution, continue stirring for 0.5h, then cool the reaction solution to room temperature, extract with ethyl acetate (3×20mL), anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain a crude product. The crude product was separated and purified by silica gel chromatography (eluent: n-hexane: ethyl acetate = 2:1), recrystallized from ethanol, and dried in vacuo to obtain the target product 5-DMN with a yield of about 56%.
[0034] The synthesis process is as follows:
[0035]
[0036] Target product 5-DMN: yellow solid powder. 1 H NMR (500MHz, DMSO-d...
Embodiment 2
[0038] This example provides the synthesis method of the target compound 5,3'-DMN
[0039] The synthesis process of the target compound 5,3'-DMN is as follows;
[0040]
[0041] Dissolve 1mmol of 3'-hydroxy-5,6,7,8,4'-pentamethoxyflavone in 25mL of anhydrous acetonitrile, add 8mmol of anhydrous aluminum chloride, stir at 60°C for 3h, TLC Detection of reaction endpoints.
[0042] Add 20mL of 3% hydrochloric acid solution, continue stirring for 0.5h, then cool the reaction solution to room temperature, extract with ethyl acetate (3×20mL), anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain a crude product. The crude product was separated and purified by silica gel column chromatography (eluent: n-hexane: ethyl acetate = 2:1), recrystallized from ethanol, and dried in vacuo to obtain the target product 5,3'-DMN with a yield of about 47%.
[0043] Target product 5,3'-DMN: yellow solid powder. 1 H NMR (500MHz, DMSO-d 6 )δ12.74(s,1H),9...
Embodiment 3
[0045] This example provides the synthesis method of compound 5,4'-DMN, specifically as follows:
[0046] Dissolve 1mmol of 4'-hydroxy-5,6,7,8,3'-pentamethoxyflavone in 25mL of anhydrous acetonitrile, add 8mmol of anhydrous aluminum chloride, stir at 60°C for 3h, TLC Detection of reaction endpoints.
[0047] Add 20 mL of 3% hydrochloric acid solution, continue stirring for 0.5 h, then cool the reaction solution to room temperature, extract with ethyl acetate (3×20 mL), dry over anhydrous Na2SO4, and remove the solvent under reduced pressure to obtain a crude product.
[0048] The crude product was separated and purified by silica gel chromatography (eluent: n-hexane: ethyl acetate = 2:1), recrystallized from ethanol, and dried in vacuo to obtain the target product 5,4'-DMN with a yield of about 60%.
[0049] The synthesis process is as follows:
[0050]
[0051] Target product 5,4'-DMN: yellow solid powder. 1 H NMR (500MHz, DMSO-d 6 )δ12.77(s,1H),10.06(s,1H),7.58(s,2H),7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com