Dry granulation method of dry suspension
A dry suspension and granule technology, applied in the field of medicine, can solve the problems of uniform granule molding of unfavorable materials, short production time, material bonding, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, dry preparation oseltamivir phosphate dry suspension
[0035] The present embodiment provides the prescription and preparation method of preparing 10,000 bags of oseltamivir dry suspension (specification 15mg), as follows:
[0036] The formulation composition is shown in Table 1:
[0037] Table 1 Composition of Oseltamivir Phosphate Dry Suspension
[0038]
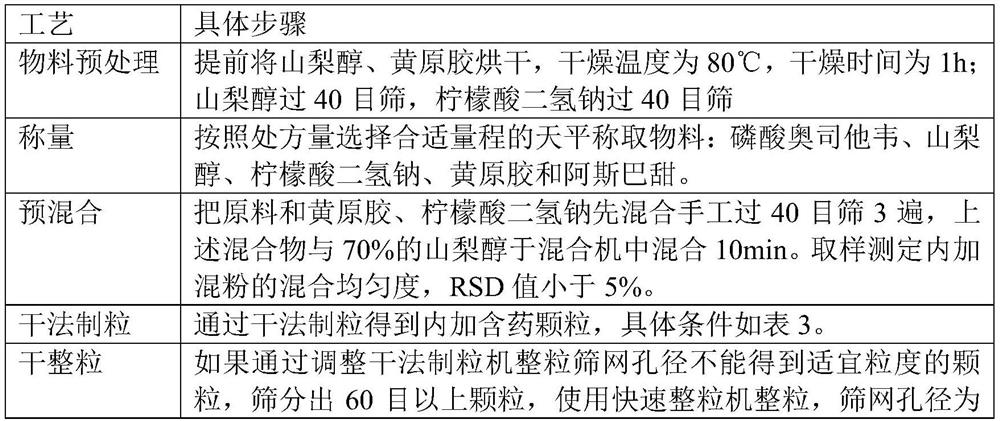
[0039] A dry granulation process was used, as shown in Table 2:
[0040] Table 2 dry granulation process steps
[0041]
[0042]
[0043] Table 3 dry granulation process conditions
[0044] Feeding screw speed 20rpm Roller speed 8rpm Roller pressure 15bar Granulator speed 100rpm Whole sieve aperture 1.5mm
Embodiment 2
[0045] Embodiment 2, dry preparation oseltamivir phosphate dry suspension
[0046] The present embodiment provides the prescription and preparation method of preparing 10,000 bags of oseltamivir dry suspension (specification 15mg), as follows:
[0047] The formulation composition is shown in Table 4:
[0048] Table 4 Composition of Oseltamivir Phosphate Dry Suspension
[0049]
[0050] A dry granulation process was used, as shown in Table 5:
[0051] Table 5 dry granulation process steps
[0052]
[0053]
[0054] The samples prepared according to this process were investigated for different xanthan gum dosages, and the results of the dissolution assay were as shown in Table 6:
[0055] The dissolution results of the oseltamivir phosphate dry suspension of the prescriptions 1-3 of table 6
[0056] Dissolution Results (%) 5min 10min 15min 20min 30min prescription 1 95 99 99 99 99 prescription 2 97 98 99 99 99 prescriptio...
Embodiment 3
[0058] Embodiment 3, dry preparation oseltamivir phosphate dry suspension
[0059] The present embodiment provides a recipe and a preparation method for preparing 10,000 bags of oseltamivir dry suspension (specification 15mg). Since the amount of xanthan gum in the recipe is small, the xanthan gum in the recipe is dried to a moisture content of less than 1 %,details as follows:
[0060] The formulation composition is shown in Table 7:
[0061] The composition of table 7 oseltamivir phosphate dry suspension
[0062]
[0063] A dry granulation process was used, as shown in Table 8:
[0064] Table 8 dry granulation process steps
[0065]
[0066]
[0067] The prepared xanthan gum is not subjected to the sample of drying treatment method for dissolution investigation and the results are as follows:
[0068] Table 10 Dissolution results of the oseltamivir phosphate dry suspensions of formulation 4 and formulation 5
[0069] Dissolution Results (%) 5min 10m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com