Preparation method of fexofenadine
A fexofenadine and solvent technology, applied in the field of chemical synthesis, can solve the problems of large environmental impact, difficult to control, difficult to recycle, etc., and achieves the effects of low cost, few reaction steps, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
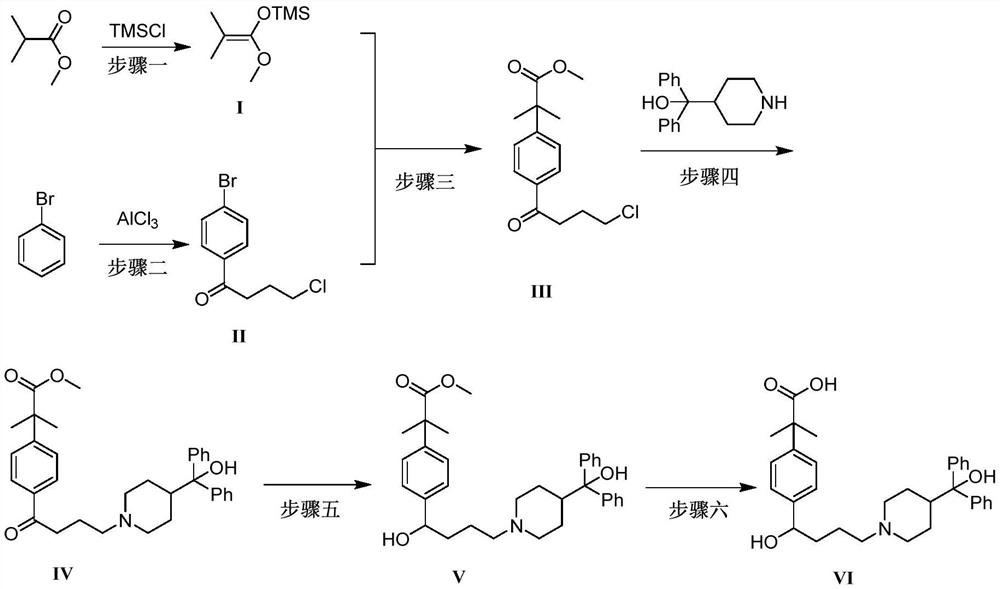
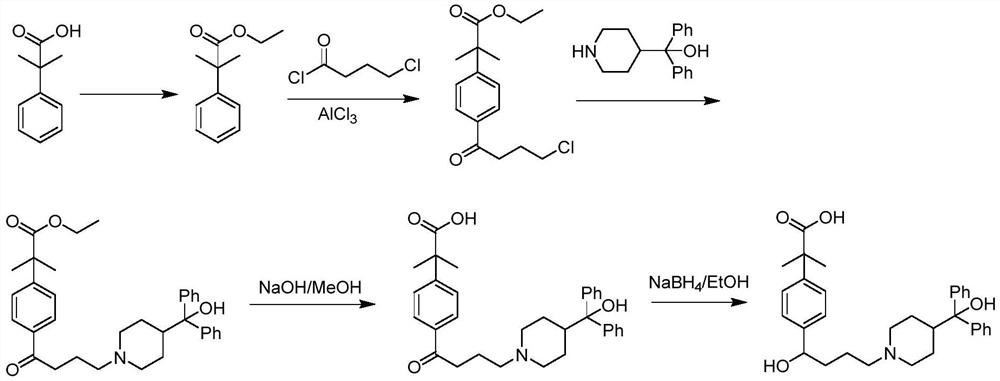
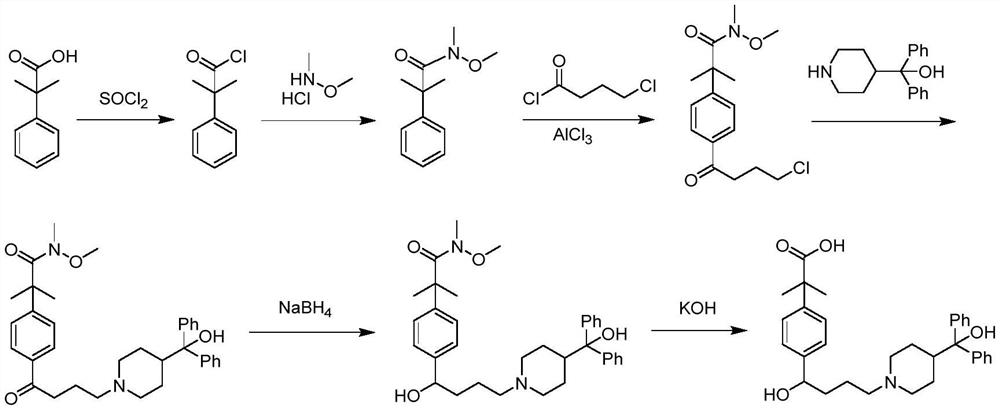
[0052]The synthesis method contains the following steps:
[0053]Step 1: Tielobutyrate is a raw material, react with trimethylchlorosilane under basic conditions under alkaline conditions to form 1-methoxy-1- (trimethylsiloxysiloxy) -2-methyl-1-propylene (I);
[0054]Step 2: Four-gramyl chloride reaction to 4-chlorocyanl chloride is carried out in a p-bromobenzene, forming 4'-bromo-4-chlorobenzine (II);
[0055]Step 3: 4'-bromo-4-chlorobenzone (II) with 1-methoxy-1- (trimethylsiloxy) -2-methyl-1-propylene (I) coupling The reaction was obtained from 2- [4- (4-chloro-1-butyl) phenyl] -2-methylpropylene (III);
[0056]Step 4: 2- [4- (4-chloro-1-butyl) phenyl] -2-methylpropylene methyl ester (III) reacts 2- [4- [4- [4 " - (Hydroxybenzyl)-1-piperidyl] -1-oxineyl] phenyl] -2,2-dimethyl acetate (IV);
[0057]Step 5: 2- [4- [4- [4- (Hydroxybenzyl)-1-piperidyl] -1-oxineyl] phenyl] -2,2-dimethyl acetate (Iv) reduction is 2- [4- [4- [4- (hydroxybenzyl)-1-piperidyl] -1-hydroxybutyl] phenyl] -2,2-dimethyl acet...
Embodiment 1
[0067]Example 1: In a nitrogen atmosphere, the methyl ester 500 g, 5L tetrahydrofuran is added to -20 ° C, slowly dropped with diisopropylamamine (3L, 2M IN THF), and dripping, shift To the room temperature reaction for 2 h, then removed to -20 ° C, add 600 g of trimethylchlorosilane, end, and moved to room temperature, there is a large amount of solid production, add n-hexane dilution, add aqueous solution, and separate liquid, collect organic phase After washing with saturated brine, the saline, dried over anhydrous sodium sulfate was dried, and the yield was obtained from a pale yellow oily liquid compound (I), and the yield was 98%.
Embodiment 2
[0068]Example 2: In a nitrogen atmosphere, methyl isobutyrate 500 g, 5L tetrahydrofuran, cooled to -20 ° C, slowly dropped the double trimethylsil-based lithium (3L, 2M in THF), and end the drop , Moved to a room temperature reaction for 2 h, then moved to -20 ° C, add 600 g of trimethylchlorosilane, end, moved to room temperature, there is a large amount of solid production, add n-hexane dilution, then add aqueous solution, and collect, collect, collect The organic phase, then washed 3 organic phases, dried over anhydrous sodium sulfate, and yields were obtained by mixed brine, and yield was 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com