A kind of method for preparing terminal 2-furanoate group polybutadiene
A technology for ester-based polybutadiene and hydroxyl-terminated polybutadiene is applied in the field of preparing 2-furocarboxylate-terminated polybutadiene, which can solve the problems of contact toxicity, rash and the like, and achieves low cost and high reaction efficiency. High activity and the effect of improving the efficiency of esterification reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take 10 g of hydroxyl-terminated polybutadiene, and dissolve 1.19 g (1.3 equivalents) of 4-dimethylaminopyridine in 40 mL of dry dichloromethane. Stir at room temperature for 10 minutes to completely dissolve 4-dimethylaminopyridine and hydroxyl-terminated polybutadiene, slowly add 0.88 mL (1.2 equivalents) of 2-furoyl chloride in an ice bath, remove the ice bath, and stir at room temperature for 5 hours. The reaction system was washed 2 to 3 times with saturated ammonium chloride solution, most of the solvent in the organic phase was removed under reduced pressure, the sample was applied by dry method, and the column chromatography separated to obtain a light yellow transparent viscous liquid terminal 2-furanoate-based polybutylene Alkene 4.2g, yield 39%.
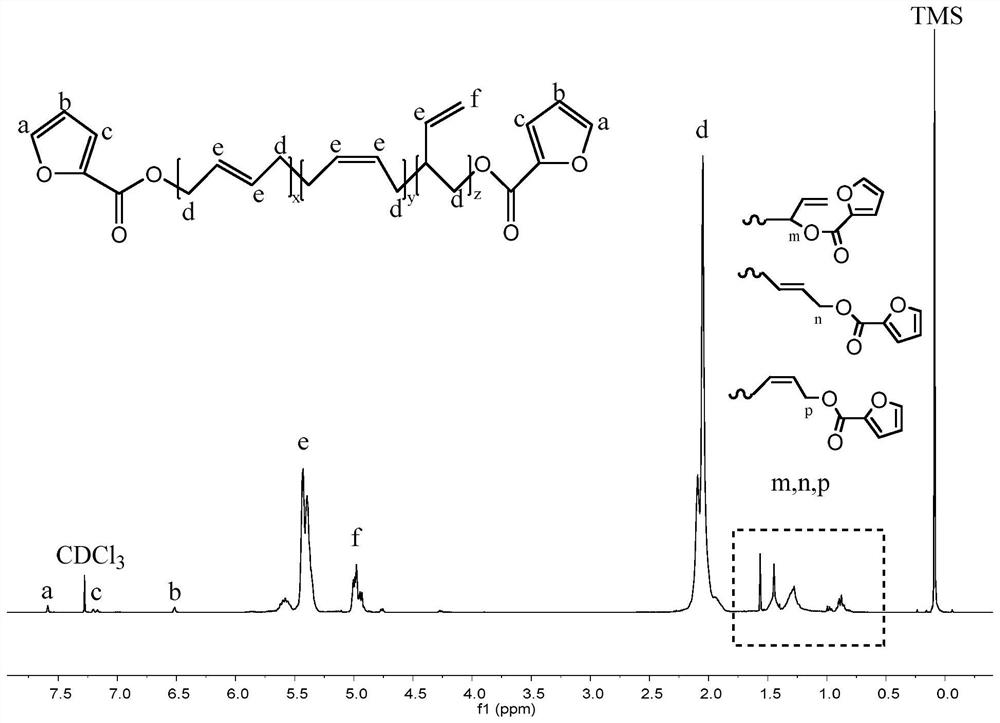
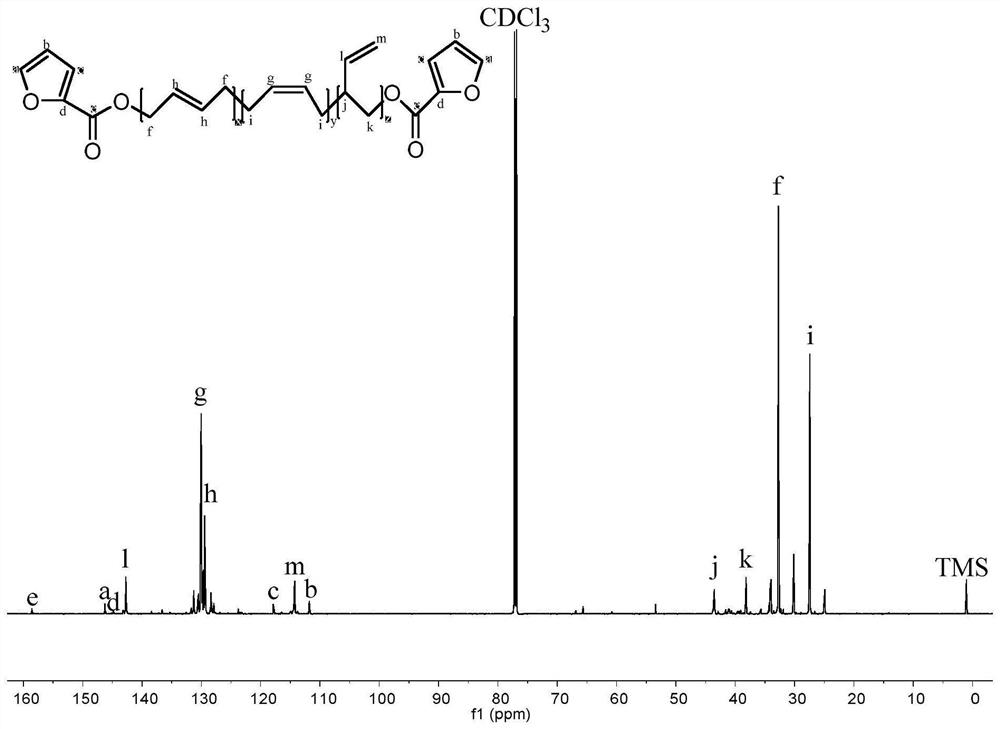
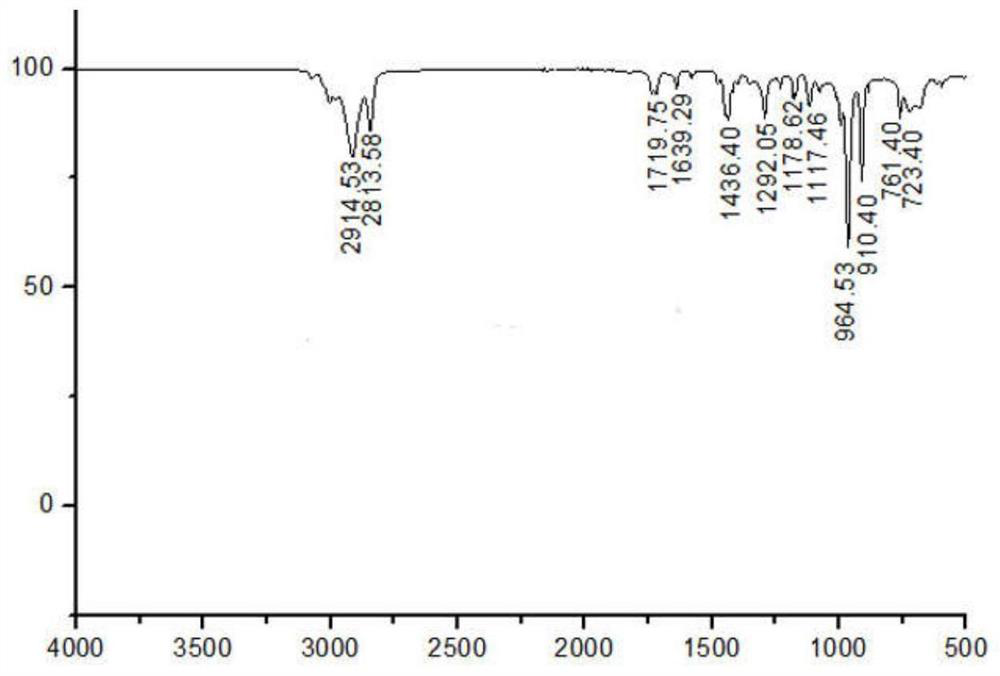
[0027] figure 1 Among them, 1.9-2.2ppm corresponds to the hydrogen atom on the methylene group connected to the olefin in the polybutadiene chain; 4.9-5.0ppm and 5.3-5.5ppm respectively correspond to the two types ...
Embodiment 2
[0032] Take 10 g of hydroxyl-terminated polybutadiene, and dissolve 1.37 g (1.5 equivalents) of 4-dimethylaminopyridine in 40 mL of dry dichloromethane. Stir at room temperature for 10 minutes to completely dissolve 4-dimethylaminopyridine and hydroxyl-terminated polybutadiene, slowly add 0.89ml (1.2 equivalents) of 2-furoyl chloride in an ice bath, remove the ice bath, and stir for 5 hours at room temperature. The reaction system was washed 2 to 3 times with saturated ammonium chloride solution, most of the solvent in the organic phase was removed under reduced pressure, the sample was applied by dry method, and the column chromatography separated to obtain a light yellow transparent viscous liquid terminal 2-furanoate-based polybutylene Alkene 7.6g, yield 71%.
Embodiment 3
[0034] Take 10 g of hydroxyl-terminated polybutadiene, and dissolve 1.83 g (2.0 equivalents) of 4-dimethylaminopyridine in 40 mL of dry dichloromethane. Stir at room temperature for 10 minutes to completely dissolve 4-dimethylaminopyridine and hydroxyl-terminated polybutadiene, slowly add 1.11 mL (1.5 equivalents) of 2-furoyl chloride in an ice bath, remove the ice bath, and stir at room temperature for 5 hours. The reaction system was washed 2 to 3 times with saturated ammonium chloride solution, most of the solvent in the organic phase was removed under reduced pressure, the sample was applied by dry method, and the column chromatography separated to obtain a light yellow transparent viscous liquid terminal 2-furanoate-based polybutylene Alkene 6.2g, yield 58%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com