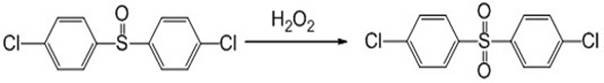
Method for synthesizing 4, 4 '-dichlorodiphenyl sulfone by using sulfoxide oxidation method
A technology of dichlorodiphenyl sulfone and dichlorodiphenyl sulfoxide, applied in 4 fields, can solve the problems of serious odor of glacial acetic acid, air pollution, etc., and achieve the effects of low cost, less usage, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 135.58 grams (0.5 moles) of 4,4'-dichlorodiphenyl sulfoxide, 135.5 grams of dichloropropane, 130 grams of water, and 8.06 grams of tetrabutylammonium bromide into a 1000ml four-necked flask with a thermometer and a condenser. After the feeding is completed, the temperature is raised to 50°C, and 188.9 grams of hydrogen peroxide with a content of 27% is slowly added dropwise for about 40 minutes-1 hour. After completion, the temperature is kept at this temperature for 7 hours, sampling is carried out, and the content is measured by liquid chromatography. 4,4'-di Chlorodiphenylsulfoxide is less than 0.3%, stop the reaction, cool to 0-5°C, filter, wash the filter cake with water, and dry to obtain 138.5g of 4,4'-dichlorodiphenylsulfone with a content of 99.5% and a yield of 96%.
Embodiment 2
[0035] Add 135.58 grams (0.5 moles) of 4,4'-dichlorodiphenyl sulfoxide, 135.5 grams of dichloropropane, 130 grams of water, and 0.81 grams of tetrabutylammonium bromide into a 1000 ml four-necked flask with a thermometer and a condenser. After the feeding is completed, the temperature is raised to 90°C, and 53.44 grams of hydrogen peroxide with a content of 35% is slowly added dropwise for about 40 minutes-1 hour. After completion, the temperature is kept at this temperature for 6 hours, and samples are taken, and the content is measured by liquid chromatography. 4,4'-di Chlorodiphenyl sulfoxide is less than 0.3%, stop the reaction, cool to 0-5°C, filter, wash the filter cake, and dry to obtain 138.8g of 4,4'-dichlorodiphenyl sulfone, content 99.1%, yield 95.8%.
Embodiment 3
[0037] Add 135.58 grams (0.5 moles) of 4,4'-dichlorodiphenyl sulfoxide, 120 grams of dichloropropane, 120 grams of water, and 3.22 grams of tetrabutylammonium bromide into a 1000ml four-necked flask with a thermometer and a condenser. After feeding, the temperature was raised to 80°C, and 99.6 grams of hydrogen peroxide with a content of 35% was slowly dropped, and the drop was completed in about 40 minutes to 1 hour. After completion, the temperature was kept at this temperature for 3 hours, and samples were taken, and the content was measured by liquid chromatography. 4,4'-di Chlorodiphenyl sulfoxide has been less than 0.4%, stop the reaction. Cool to 0-5°C, filter, wash the filter cake twice, and dry to obtain 137.8g of 4,4'-dichlorodiphenylsulfone, content 99.0%, yield 95.0%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com