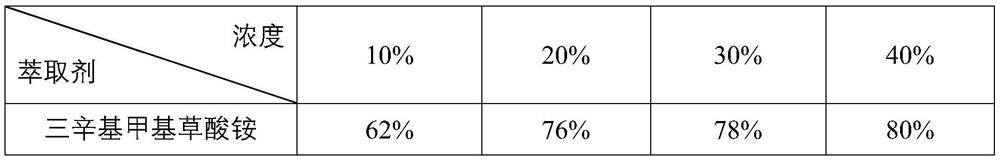
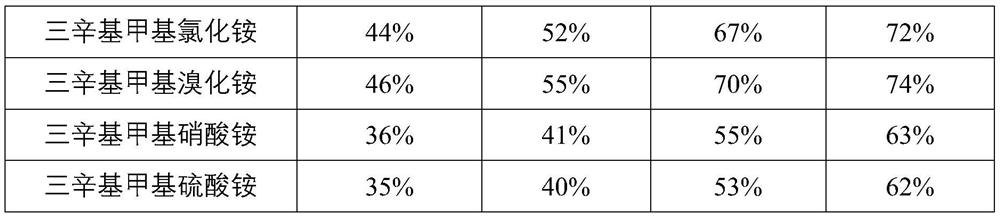
Preparation method of trioctyl methyl ammonium oxalate
A technology of trioctylmethylammonium oxalate and trioctylmethylammonium chloride, which is applied in the field of preparation of trioctylmethylammonium oxalate, can solve the problems of weak extraction ability, high solvent content, polluted wastewater, etc. Achieve the effect of purity, green environmental protection, strong extraction ability, and avoid pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The invention provides a preparation method of trioctylmethylammonium oxalate.
[0018] The method (1) mixes trioctyl tertiary amine and isooctyl alcohol, puts it in a vertical reactor, then continuously feeds methyl chloride at a rate of 100-300mL / min, and reacts at 100-200°C for 5- 10h, obtain trioctyl methyl ammonium chloride crude product;
[0019] (2) The crude product of trioctylmethyl ammonium chloride is transferred to a falling film liquid film distillation apparatus, and distilled at 120~150°C and 2~8mmHg column conditions to obtain trioctylmethyl ammonium chloride;
[0020] (3) Mix trioctylmethyl ammonium chloride with wet silver oxide, place in a reaction kettle, stir at 40-80°C for 2-6 hours, cool to room temperature and separate phases to obtain trioctylmethyl ammonium chloride Ammonium hydroxide;
[0021] (4) Mix trioctylmethylammonium hydroxide and oxalic acid, place in a reaction kettle, stir at 30-60°C for 2-6h, cool to room temperature and separate ...
Embodiment 1
[0033] A method for synthesizing trioctylmethylammonium oxalate. The synthetic method of trioctyl methyl ammonium oxalate described in the present embodiment is:
[0034] Step 1. According to the volume ratio of 1: (1.5~2), trioctyl tertiary amine and isooctyl alcohol are mixed, placed in a vertical reactor, and then continuously fed into methyl chloride at a speed of 100~200mL / min, React at 100-150°C for 5-8 hours to obtain the crude product of trioctylmethylammonium chloride;
[0035] Step 2, transfer the crude product of trioctylmethyl ammonium chloride to a falling film liquid film distillation apparatus, and distill at 120~150°C and 2~6mm (Hg column) conditions to obtain trioctylmethyl ammonium chloride Ammonium;
[0036] Step 3. According to the molar ratio of 1: (1-1.5), mix trioctylmethylammonium chloride with wet silver oxide, place in a reaction kettle, stir at 40-60°C for 2-4 hours, and cool After reaching room temperature, the phases were separated to obtain tri...
Embodiment 2
[0044] A method for synthesizing trioctylmethylammonium oxalate. The synthetic method of trioctyl methyl ammonium oxalate described in the present embodiment is:
[0045] Step 1. According to the volume ratio of 1: (2~2.5), trioctyl tertiary amine and isooctyl alcohol are mixed, placed in a vertical reactor, and then continuously fed into methyl chloride at a speed of 150~250mL / min, React at 130-180°C for 6-9 hours to obtain the crude product of trioctylmethylammonium chloride;
[0046] Step 2, transfer the crude product of trioctylmethyl ammonium chloride to a falling film liquid film distillation apparatus, and distill at 120~150°C and 3~7mm (Hg column) conditions to obtain trioctylmethyl ammonium chloride Ammonium;
[0047] Step 3. According to the molar ratio of 1: (1.5-2.5), mix trioctylmethylammonium chloride with wet silver oxide, place in a reaction kettle, stir at 50-70°C for 3-5 hours, and cool After reaching room temperature, the phases were separated to obtain tri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com