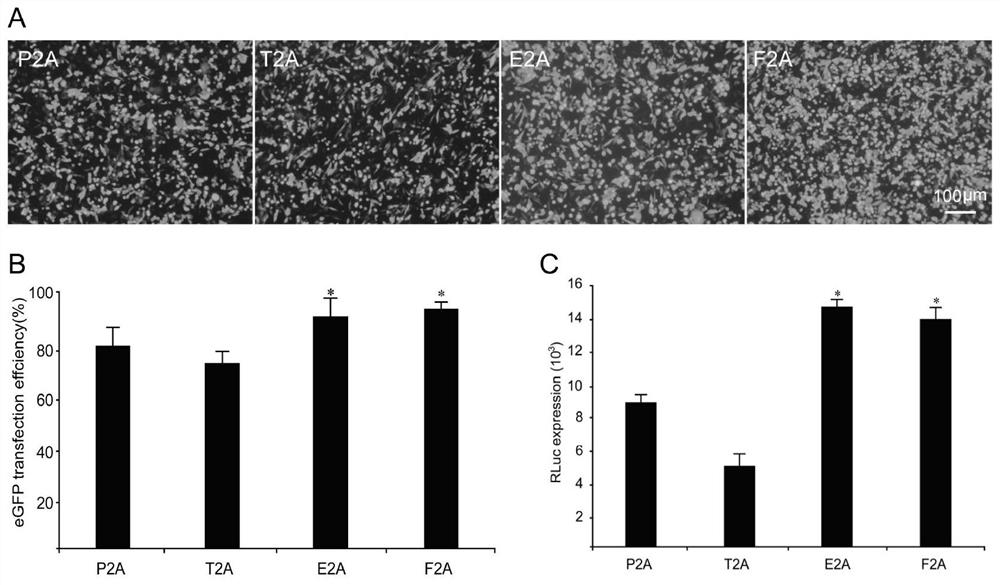
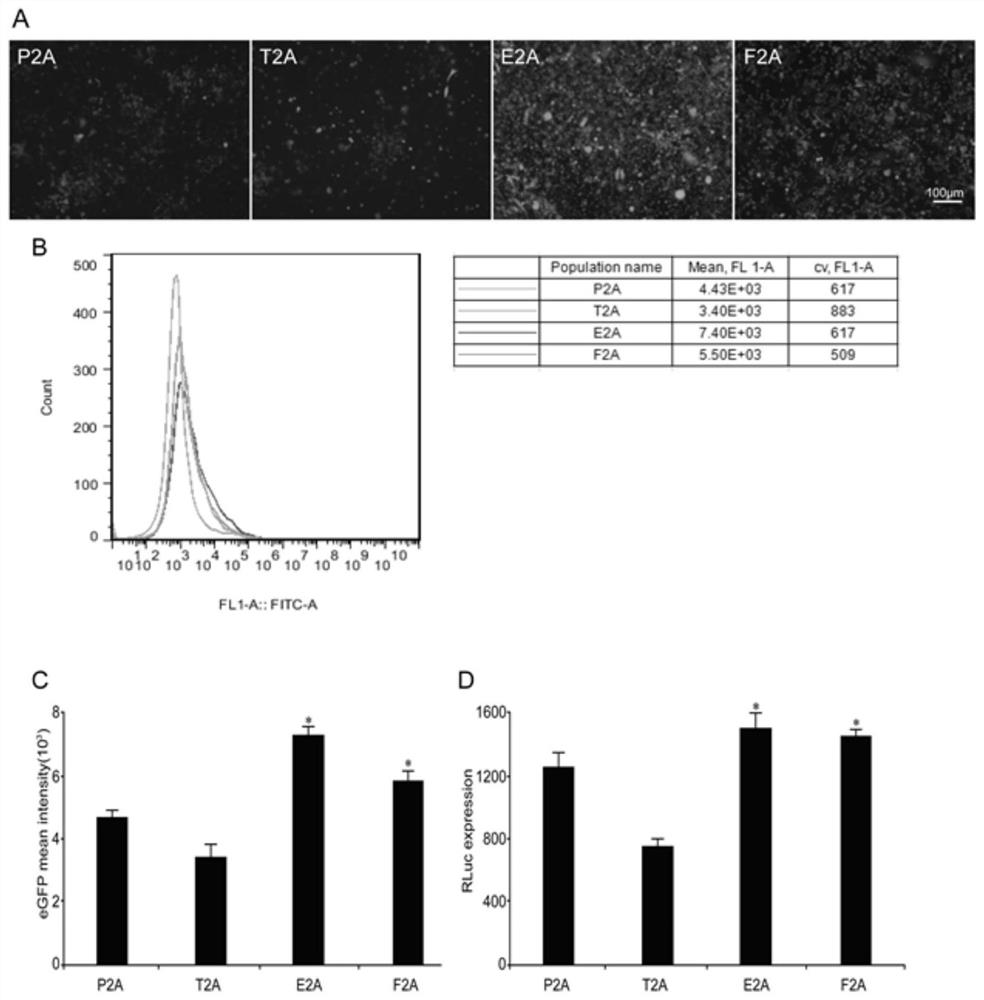
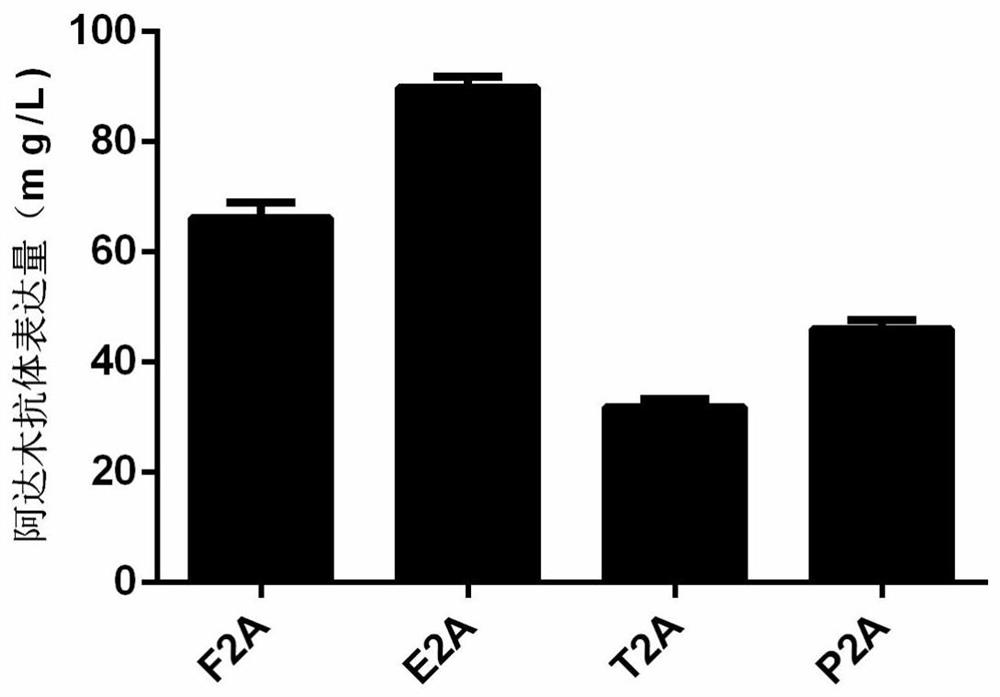
2A peptide, bicistronic expression vector, recombinant protein expression system and application
A technology of recombinant protein and expression vector, which is applied in the field of genetic engineering, can solve the problems of low expression level of target gene and inability to translate downstream genes, etc., achieve high-efficiency expression, increase expression amount, and reduce production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Synthesis of 2A peptide gene
[0033] This example provides the gene sequence for the 2A peptide.
[0034] According to the amino acid sequence of foot-and-mouth disease virus (F2A) shown in SEQ ID NO.1, and the equine rhinitis A virus (equine rhinitis A virus, E2A) shown in SEQ ID NO.3, such as SEQ ID Brown winged moth virus (Thosea asigna virus, T2A) shown in NO.5 and porcine Teschovirus (porcine teschovirus, P2A) amino acid sequence shown in SEQ ID NO.7; According to CHO cell codon preference, Design the 2A peptide gene sequence, as shown in SEQ ID NO.2, SEQ ID NO.4, SEQ ID NO.6, and SEQ ID NO.8.
Embodiment 2
[0035] Example 2 Construction of recombinant expression vector containing 2A peptide gene
[0036] This embodiment provides a method for constructing a recombinant expression vector comprising a gene encoding a 2A peptide, comprising the following steps:
[0037] Artificially synthesized SEQ ID NO.2, SEQ ID NO.4, SEQ ID NO.6, and SEQ ID NO.8. In order to realize directional cloning, StuI (AGGCCT) and AgeI were introduced at the 5' end and 3' end of the synthetic sequence respectively. (ACCGGT) restriction site.
[0038] The synthesized 2A sequence was double-digested with AgeI / StuI respectively, and the pIRES-Neo plasmid DNA vector was double-digested with AgeI / StuI at the same time. The digestion results were identified by agarose gel electrophoresis, and the digested 2A sequence fragment and pIRES-Neo linear plasmid DNA were recovered from the gel.
[0039]The double enzyme digestion system for 2A sequence is: 10 μL of 2A sequence (1 μg / μL), 3.0 μL of 10×CutSmart Buffer, 1...
Embodiment 3
[0042] Example 3 Construction of EGFP recombinant expression vector
[0043] The present embodiment provides the construction method of the engineered bacterium that contains recombinant expression vector, comprises the following steps:
[0044] 1) PCR amplification of EGFP gene
[0045] Referring to the Enhanced green fluorescent protein (EGFP) gene sequence of the pEGFP-C1 vector (GenBank: U55763.1, bases 613-1332), design primers P1 and P2 (for amplifying 720bp EGFP gene DNA), primer 5 EcoRV and NheI restriction sites were introduced at the ' ends respectively, and the primer sequences were as follows (the restriction sites are underlined):
[0046] P1: 5′-CCG GATATC ATGGTGAGCAAGGGCGAGGAG-3′;
[0047] P2: 5′-CTA ACCGGT GGACTTGTACAGCTCGTCCATGC-3'.
[0048] Using the pEGFP-C1 plasmid (purchased from Clontech, USA) as a template, primers P1 and P2 were used to amplify the EGFP gene. The reaction system is shown in Table 1 below.
[0049] Table 1 PCR amplification syste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com