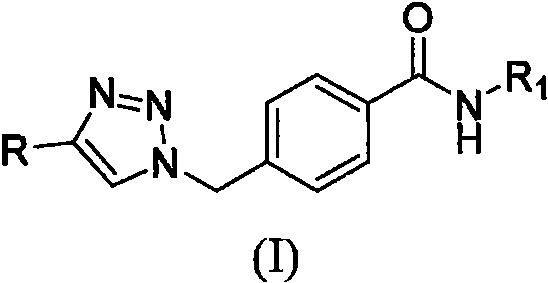
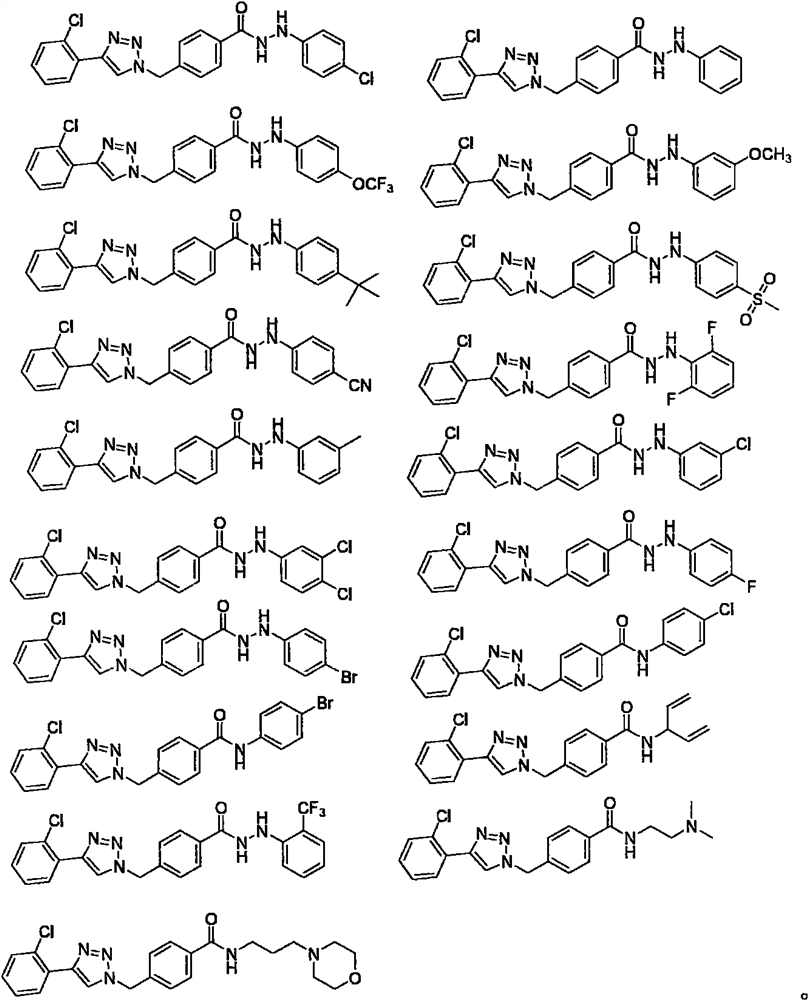
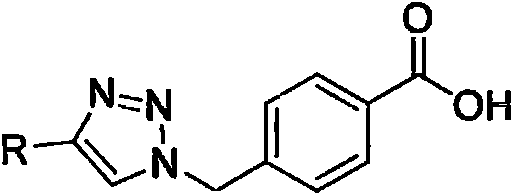
1, 2, 3-triazole hydrazide or amide compound as well as preparation method and application thereof
A compound and composition technology, applied in 1 field, can solve the problems of reduced potency, complicated situation of multidrug-resistant microbial strains, increased number of infections, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the preparation of intermediate 2 (4-(azidomethyl) methyl benzoate)
[0055] The raw material 4-bromomethylbenzoic acid methyl ester (5.00g, 21.83mmol) and sodium azide (7.10g, 0.11mol) were added in a 100mL reaction flask, and 14mL N,N-dimethylformamide ( DMF), heated to 60°C for 4 hours to stop the reaction, extracted with ethyl acetate, washed with saturated ammonium chloride three to four times, dried over anhydrous sodium sulfate, and precipitated to obtain 4.12 g of a colorless oil, with a yield of 98.80% .
Embodiment 2
[0056] Example 2: Preparation of intermediate 3 (4-((4-(2-chlorophenyl)-1H-1,2,3-triazol-1-yl)methyl)methyl benzoate)
[0057]Intermediate 2 (4.0g, 20.92mmol) and 1-chloro-2-ethynylbenzene (2.60g, 19.02mmol) were dissolved in 10 mLTHF, then 10mL of water was added to the system, and anhydrous copper sulfate (0.48g , 1.90mmol) and sodium ascorbate (0.75g, 3.80mmol) were dissolved in 8mL of water and added dropwise to the above system, reacted at room temperature for 14 hours, then stopped the reaction, precipitated, dissolved in water, then extracted with dichloromethane, anhydrous Drying over sodium sulfate, precipitation, and separation by column chromatography gave 6.24 g of a white solid with a yield of 99.39%. Its NMR data are: 1 H NMR (500MHz, CDCl 3 ) δ8.23 (dd, J=7.9, 1.7Hz, 1H, 2-Cl-phenyl-H), 8.15 (s, 1H, triazole-H), 8.03-8.00 (m, 2H, benzene-H), 7.40(dd, J=8.0, 1.3Hz, 1H, 2-Cl-phenyl-H), 7.36-7.33(m, 2H, benzene-H), 7.33-7.31(m, 1H, 2-Cl-phenyl- H), 7.24 (ddd, J...
Embodiment 3
[0058] Example 3: Preparation of intermediate 4 (4-((4-(2-chlorophenyl)-1H-1,2,3-triazol-1-yl)methyl)benzoic acid)
[0059] Intermediate 3 (4.30g, 13.12mmol) was dissolved in 20mL of tetrahydrofuran, and then 20mL of water dissolved in KOH (1.10g, 19.68mmol) was added to the system, and heated to 55°C for 3 hours to stop the reaction and desolvate. The pH was adjusted to 3-4 with dilute hydrochloric acid, a large amount of solids were precipitated, and filtered by suction to obtain 3.85 g of white solids, with a yield of 93.39%. Its NMR data are: 1 H NMR (400MHz, DMSO-d6) δ13.04(s, 1H, -COOH), 8.82(s, 1H, triazole-H), 8.09(dd, J=7.8, 1.8Hz, 1H, 2-Cl-phenyl -H), 7.96(d, J=8.3Hz, 2H, benzene-H), 7.56(dd, J=7.9, 1.3Hz, 1H, 2-Cl-phenyl-H), 7.4 8-7.44(m, 2H , benzene-H), 7.44 (d, J=2.5Hz, 1H, 2-Cl-phenyl-H), 7.41-7.35 (m, 1H, 2-Cl-phenyl-H), 5.80 (s, 2H, CH 2 ); 13 C NMR (101MHz, DMSO-d 6 )δ167.4, 143.3, 141.3, 131.0, 130.8, 130.7, 130.3, 130.0, 129.5, 128.4, 128.0, 125.2, 53....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com