Method for preparing mesalazine enteric-coated sustained-release capsule
A technology of mesalamine and sustained-release capsules, which is applied in the field of preparation of mesalamine enteric-coated sustained-release capsules, can solve the problems of long residence time, weak digestion ability, coating failure, etc., and achieve the goal of improving the probability of cure and good drug efficacy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
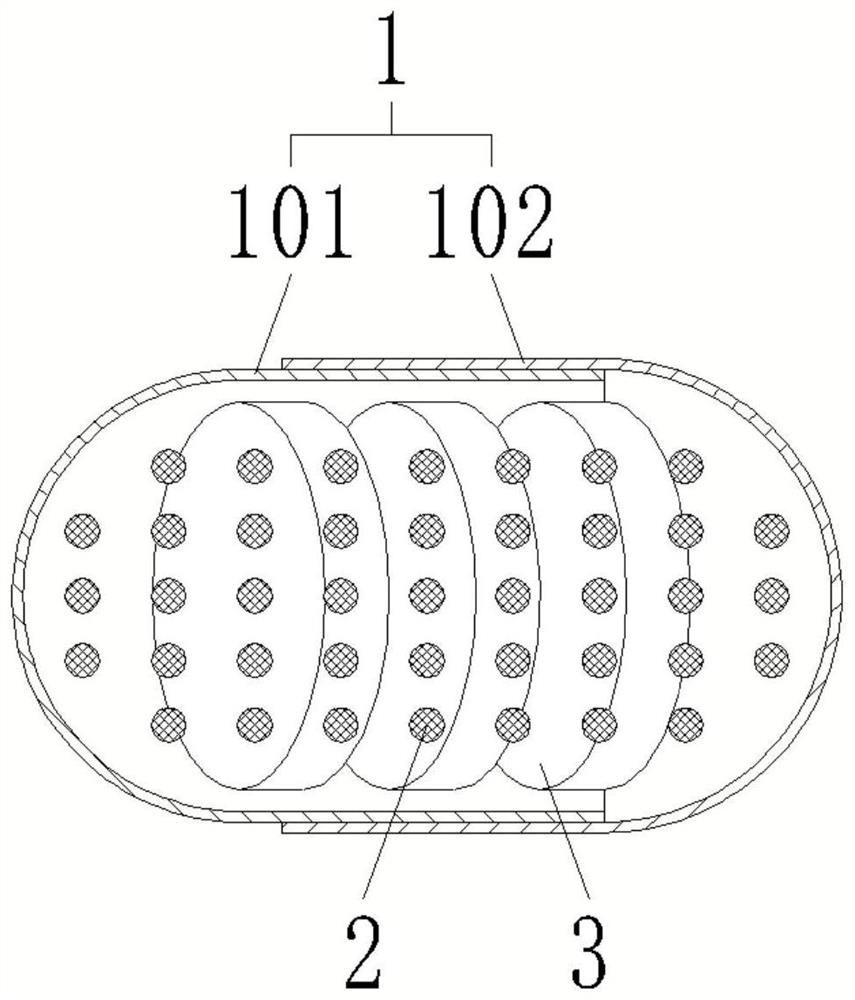
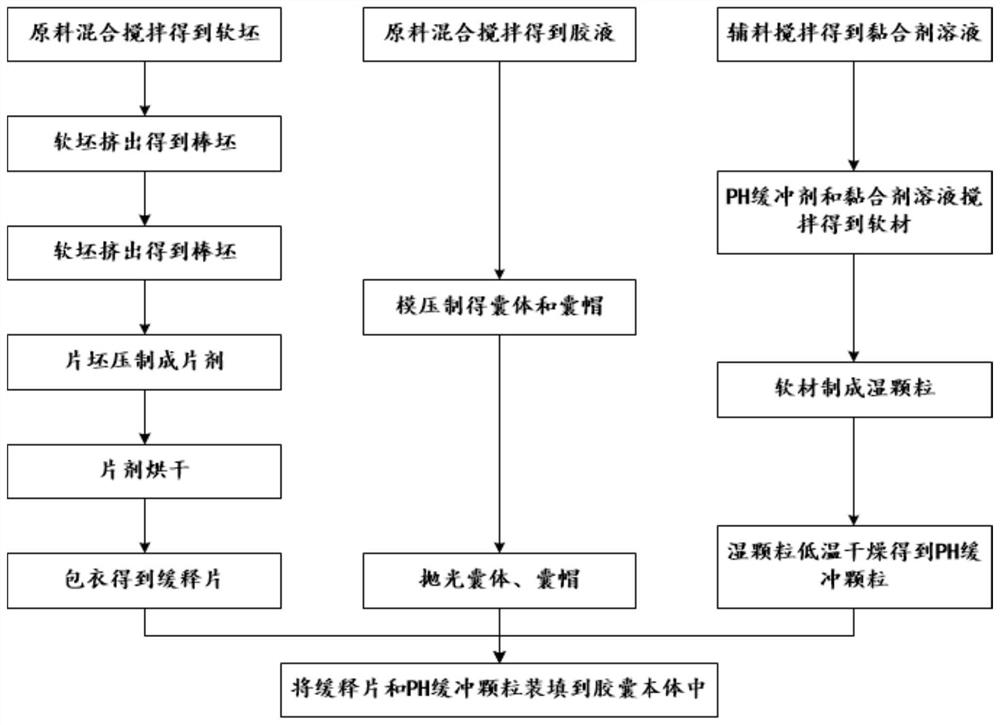
[0046] like figure 1 and 2 Shown, a kind of method for preparing mesalamine enteric-coated sustained-release capsule, mesalamine enteric-coated sustained-release capsule comprises capsule body 1 and the mesalamine enteric-coated sustained-release tablet 3 that is sealed and accommodated in capsule body 1 and The number of pH buffer granules 2 and mesalamine enteric-coated sustained-release tablets 3 in mesalamine enteric-coated sustained-release capsules is more than two. The method includes the following steps:
[0047] S1. Preparation of mesalamine enteric-coated sustained-release tablets 3. This step includes the following sub-steps:
[0048] S11, the mesalazine of 110 mass parts, the dextrin of 5 mass parts, the starch of 12 mass parts, the mannitol of 22 mass parts, the microcrystalline cellulose of 14 mass parts, the magnesium stearate of 1.8 mass parts and 100 mass parts Parts of pure water are fully mixed and stirred to obtain a soft billet.
[0049] S12, extrudin...
Embodiment 2
[0064] A method for preparing mesalamine enteric-coated sustained-release capsules, comprising the steps of:
[0065] S1. Preparation of mesalamine enteric-coated sustained-release tablets 3. This step includes the following sub-steps:
[0066] S11, the mesalazine of 120 mass parts, the dextrin of 7 mass parts, the starch of 16 mass parts, the mannitol of 26 mass parts, the microcrystalline cellulose of 16 mass parts, the magnesium stearate of 2.2 mass parts and 140 Parts by mass of pure water are fully mixed and stirred to obtain a soft billet.
[0067] S12, extruding the soft billet to obtain a billet, the diameter of the billet is 5 mm.
[0068] S13, cutting the billet into slices, the thickness of which is 3 mm.
[0069] S14, pressing the blank into a tablet, the diameter of the tablet is 6mm, and the thickness is 2mm.
[0070]S15, drying and coating the tablet to obtain the mesalazine enteric-coated sustained-release tablet 3.
[0071] S2. Prepare the capsule body 1....
Embodiment 3
[0082] A method for preparing mesalamine enteric-coated sustained-release capsules, the method comprising the steps of:
[0083] S1. Preparation of mesalamine enteric-coated sustained-release tablets 3. This step includes the following sub-steps:
[0084] S11, the mesalazine of 115 mass parts, the dextrin of 6 mass parts, the starch of 14 mass parts, the mannitol of 24 mass parts, the microcrystalline cellulose of 15 mass parts, the magnesium stearate of 2 mass parts and 120 Parts by mass of pure water are fully mixed and stirred to obtain a soft billet.
[0085] S12, extruding the soft billet to obtain a billet, the diameter of the billet is 5mm.
[0086] S13, cutting the billet into slices, the thickness of which is 3mm.
[0087] S14, pressing the blank into a tablet, the diameter of the tablet is 6mm, and the thickness is 2mm.
[0088] S15, drying and coating the tablet to obtain the mesalazine enteric-coated sustained-release tablet 3.
[0089] S2. Prepare the capsule...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com