Copper-catalyzed N-alkylated benzimidazole derivative and complex one-step in-situ synthesis method
A technology for the synthesis of benzimidazole and its synthesis method, which is applied in the field of synthesis of copper-catalyzed N-alkylated benzimidazole derivatives and complexes, can solve the problems of high cost, long reaction time, separation, etc., and achieve easy control and condition Gentle, easy-to-synthesize effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
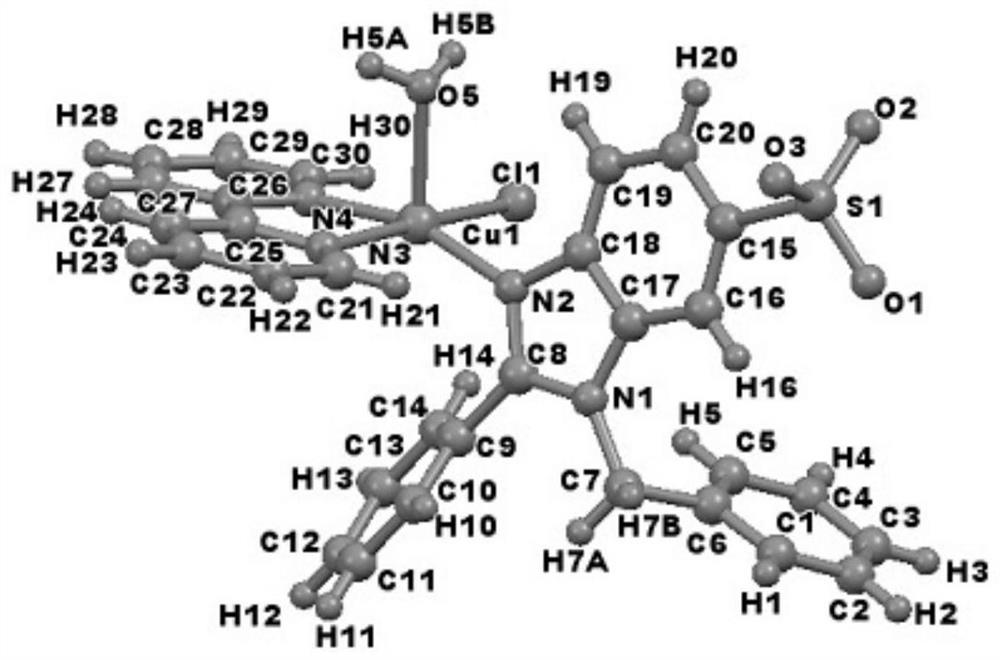
[0024] 0.2mmol catalyst CuCl 2 , 0.2mmol raw material 2-phenylbenzimidazole-5-sulfonic acid was dissolved in 10mL deionized water, added 0.2mmol NaOH and stirred at room temperature for 2h, then, 0.2mmol 2,2′-bipyridine in 10mL ethanol solution was added to the above The solution was heated to reflux at 80°C for 12h, and cooled to room temperature. It was filtered and placed at room temperature, and blue blocky crystals were obtained after 7d.
Embodiment 2
[0026] 0.3mmol catalyst CuCl 2 , 0.3mmol raw material 2-phenylbenzimidazole-5-sulfonic acid was dissolved in 10mL deionized water, added 0.3mmol NaOH and stirred at room temperature for 2h, then, 0.3mmol 2,2'-bipyridine in 10mL ethanol solution was added to the above The solution was heated to reflux at 80°C for 12h, and cooled to room temperature. It was filtered and placed at room temperature, and blue blocky crystals were obtained after 7d.
Embodiment 3
[0028] 0.4mmol catalyst CuCl 2 , 0.4mmol raw material 2-phenylbenzimidazole-5-sulfonic acid was dissolved in 10mL deionized water, added 0.4mmol NaOH and stirred at room temperature for 2h, then, 0.4mmol 2,2′-bipyridine in 10mL ethanol solution was added to the above The solution was heated to reflux at 80°C for 12h, and cooled to room temperature. It was filtered and placed at room temperature, and blue blocky crystals were obtained after 7d.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com