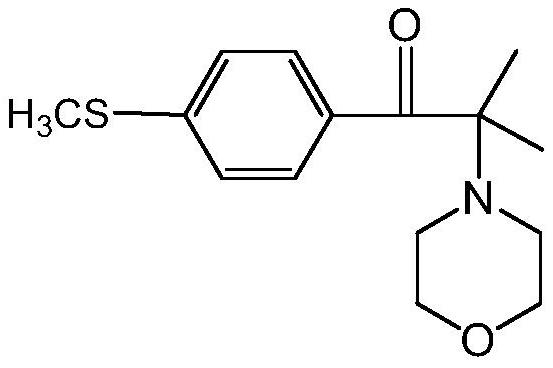
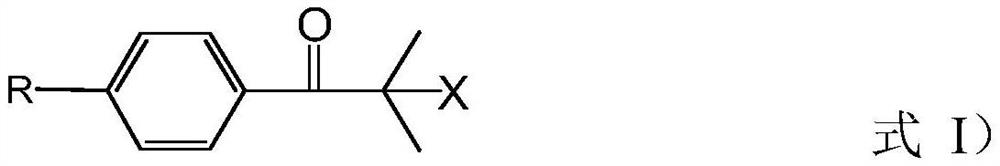Preparation method of 2-methyl-1-(4-substituted phenyl)-2-morpholinyl-1-acetone
A morpholino and morpholine technology, applied in the field of organic synthesis, can solve the problems of high operation cost, poor purification, complicated process and the like, and achieve the effect of low operation risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] This embodiment provides a preparation method of 2-methyl-1-(4-methylthiophenyl)-2-morpholino-1-propanone, which specifically includes the following steps:
[0076] (a) add 2000L methyl alcohol, 535kg morpholine and 710kg sodium methylate in reactor, obtain the mixed solution of methyl alcohol, morpholine and sodium methylate; Cool down to temperature and be 28 ℃, slowly drop 1000kg bromide in above-mentioned mixed solution, After the dropwise addition, the temperature was controlled at 28°C for 0.5h; then under normal pressure, the temperature was raised to 85°C for methanol distillation; the molecular formula of the bromide was as follows:
[0077]
[0078] (b) add 1450kg morpholine, 140kg water in the reactor of step (a); Insulation 105 ℃ of reaction 12h;
[0079] (c) The product in step (b) is distilled morpholine under reduced pressure at a vacuum pressure of -0.05MPa and a temperature of 100°C; then add 3000L of toluene, wash twice with 350L of water, and separ...
Embodiment 2
[0085] The difference between this embodiment and Example 1 is that in step (b), 140kg of water is replaced with 50L concentration of sodium carbonate aqueous solution of 0.1mol / L, and other parameters and conditions are exactly the same as in Example 1.
[0086]Purity test was carried out on the above product, the product purity was 99.0%, and the yield was 90%.
Embodiment 3
[0088] The difference between this embodiment and Example 1 is that in step (b), 140kg of water is replaced with 30L concentration of sodium bicarbonate aqueous solution of 0.1mol / L, and other parameters and conditions are exactly the same as in Example 1.
[0089] Purity test was carried out on the above product, the product purity was 99.1%, and the yield was 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com